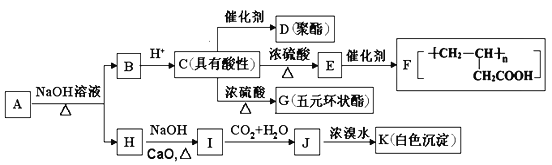
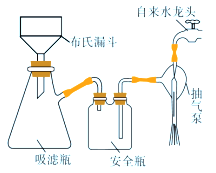
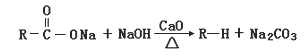��Ŀ����
����Ŀ��25��ʱ�� c mol��L��1CH3COOH ��Һ��ˮϡ�ͣ� ��Һ�� CH3COOH �� CH3COO�������и�����ռ�����ʵ�������(��)����Һ pH �仯�Ĺ�ϵ��ͼ��ʾ������˵������ȷ����
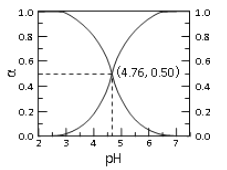
A. ��ͼ��֪�� 25��ʱ����� Ka=10-4.76
B. c mol��L��1CH3COOH ��Һ��ˮϡ���У� ��(CH3COOH)���� c(CH3COO��)Ҳһ������
C. �� pH��4.76 ����Һ��ͨ�� HCl�� ��(CH3COOH)������(CH3COO��)��С�� ��(CH3COOH)+��(CH3COO��)=1
D. ����ͼ��������������һ������Ӧ����Һ�У����� c(CH3COO��)+c(OH��)=c(H+)
���𰸡�B
��������A. ��ͼ��֪��pH=4.76ʱ����(CH3COOH)=c(CH3COO��) ������25��ʱ����� Ka=10-4.76��A��ȷ��B. c mol��L��1CH3COOH ��Һ��ˮϡ���У�CH3COOH�ĵ���ƽ�������ƶ���������(CH3COOH)��С�� c(CH3COO��)������B����ȷ��C. �� pH��4.76 ����Һ��ͨ�� HCl��HCl����ʹ��Һ��c(H+)����CH3COOH�ĵ���ƽ�������ƶ� ��������(CH3COOH)������(CH3COO��)��С�����������غ��֪�� ��(CH3COOH)+��(CH3COO��)=1��C��ȷ��D. ���������غ��֪������ͼ��������������һ������Ӧ����Һ�У����� c(CH3COO��)+c(OH��)=c(H+)��D��ȷ������ѡB.

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�