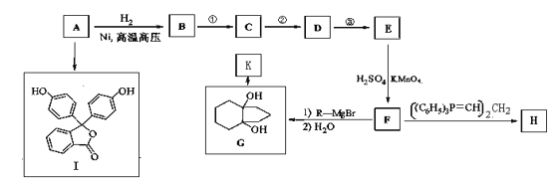
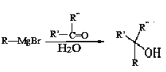
��Ŀ����
����Ŀ�����ǵؿ��к������Ľ���Ԫ�أ������仯�������ճ������ҵ���й㷺��Ӧ�á�
(1)��ԭ�Ӻ�����_________�ֲ�ͬ�˶�״̬�ĵ��ӣ���_________��������ͬ�ĵ��ӣ�д������Ԫ�����ڱ��е�λ�ã�________
(2)����������ǿ�ȸߣ���ĥ������ʴ���۵�ɴ�2200�档�Ʋ������________���壬�ԱȽ���ɸ����ʵ������뾶��С��_______
(3)����������������Ӧ���Ʊ���������4BaO+2Al![]() BaO��Al2O3+3Ba������Ҫԭ����_______(ѡ����)��
BaO��Al2O3+3Ba������Ҫԭ����_______(ѡ����)��
a.Al�����Դ���Ba b.Ba�е��Al�ĵ� c.BaO��Al2O3��Al2O3�ȶ�
(4)��ҵ��������������������̼������ȡ����ʯ(Na3AlF6)���䷴Ӧ����������Ԫ�������ڱ���λ�����ڣ��ɱȽ����ǽ����Ի�ǽ�����ǿ������_________(ѡ����)��
a.��̬�⻯����ȶ��� b.����������Ӧˮ�������(��)��
c.������������Ӧ������ d.������ͬŨ���ᷢ����Ӧ�Ŀ���
(5)������ҵ�ϲ��õ���Ȼ��������õ���������ķ�����������ʵ�ԭ��_______
���𰸡�13 5 �������ڢ�A�� ԭ�� Al>N b ac �Ȼ���Ϊ���Ӿ��壬�۵���Ҳ����룬��������Ϊ���Ӿ���
��������
(1)���κ�ԭ���ж��������˶�״̬��ȫ��ͬ�ĵ��ӣ����ԭ�Ӻ�������Ų�ʽȷ����ԭ�Ӻ������������������Ŀ��
(2)ԭ�Ӿ��壬Ӳ�ȴ��۵�ߣ����ӵĵ��Ӳ�Խ�࣬���ӵİ뾶Խ��
(3)������Al�Ľ����Ա�Ba�Ľ����������÷�Ӧ������Ba�ķе��Al�ĵͣ�
(4)���ݷ�Ӧ���е�Ԫ�ؿ�֪��������Ԫ��λ�����ڣ������÷ǽ�����ǿ�����жϷ��������
(5)�Ȼ���Ϊ���ۻ���������в��������ӣ�����ʱ���ܵ��硣
(1)����13��Ԫ�أ����������13����ÿһ�����ӵ��˶�״̬����ͬ����������Ų�ʽΪ1s22s22p63s23p1����5���ܼ��������5��������ͬ�ĵ��ӣ���ԭ�ӵĺ�������Ų�Ϊ2��8��3����������Ԫ�����ڱ���λ�ڵ������ڢ�A�壻
(2)ԭ�Ӿ���Ӳ�ȴ��۷е�ߣ����ݵ��������������ʣ�����Ӳ�ȴ��۵�ߡ���ѧ�����ȶ�����֪����������ԭ�Ӿ��壬AlԪ��ԭ�Ӻ��������Ϊ13����3�����Ӳ㣬NԪ��ԭ�Ӻ��������Ϊ7����2�����Ӳ㣬ԭ�Ӻ�����Ӳ�Խ��ԭ�Ӱ뾶Խ���������뾶��СAl>N��
(3)����Ԫ��Ba��Al��Ԫ�����ڱ���λ�ÿ�֪���������ԣ�Al<Ba����Al�ڸ����¿ɽ��������б��û�������������Ba�ķе�����ĵͣ�����ʱBaת��Ϊ�������뷴Ӧ��ϵ���Ӷ�ʹ���淴Ӧ������У�������ȡ�õ�����Ba���ʺ���ѡ����b��
(4)�÷�Ӧ�е����ʺ��е�Ԫ����Al��O��H��F��Na��C��ֻ��O��FԪ�����ڣ���F�ķǽ�������ǿ��û�����ۣ�Ҳ��û������������Ӧˮ�������Ҳ�����ᷴӦ��������������̬�⻯����ȶ��Ժ͵�����������Ӧ���������ж�O��F�ǽ����Ե�ǿ�����ʺ���ѡ����Ϊac��
(5)��Ϊ�Ȼ���Ϊ���ۻ�����ɷ��ӹ��ɣ����ڷ��Ӿ��壬�����в��������ӣ�����ʱ���ܵ��磬�ʲ��ܱ���⣻��������Ϊ���ӻ��������״̬���Ե������Al3+��O2-���ܵ��磬Al3+�������ϵõ����ӱ�Ϊ����Al��

 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д�����Ŀ�������ֽ�ȿɷ��ΰ�����Ⱦ�����ܵõ�����Դ���õ��㷺�о���
(1)��֪���ٷ�ӦI��4NH3(g)+3O2(g)![]() 2N2(g)+6H2O(g) ��H1=-1266.6 kJ��mol-1
2N2(g)+6H2O(g) ��H1=-1266.6 kJ��mol-1
��H2(g)+![]() O2(g)=H2O(l) ��H2=-285.8 kJ��mol-1
O2(g)=H2O(l) ��H2=-285.8 kJ��mol-1
��H2O(l)�TH2O(g) ��H3=+44.0 kJ��mol-1
��Ӧ2NH3(g)![]() N2(g)+3H2(g)�ķ�Ӧ����H=___��
N2(g)+3H2(g)�ķ�Ӧ����H=___��
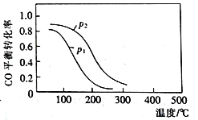
(2)�ϳɼ״��ķ�ӦΪ��CO(g)+2H2(g)![]() CH3OH(g) ��H2����10 L�����ܱ������м���4 mol CO��8 mol H2�����CO��ƽ��ת�������¶Ⱥ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��200��ʱn(H2)��ʱ��ı仯���±���ʾ��
CH3OH(g) ��H2����10 L�����ܱ������м���4 mol CO��8 mol H2�����CO��ƽ��ת�������¶Ⱥ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��200��ʱn(H2)��ʱ��ı仯���±���ʾ��
t/min | 0 | 1 | 3 | 5 |
n(H2)/mol | 8.0 | 5.4 | 4.0 | 4.0 |
�١�H2_____(���������������)0��
������˵����ȷ����_______(���ţ���
a.�¶�Խ�ߣ��÷�Ӧ��ƽ�ⳣ��Խ��
b.��ƽ����ٳ���ϡ�����壬CO��ת�������
c.����������ѹǿ���ٱ仯ʱ����Ӧ�ﵽ�����
d.ͼ��ѹǿp1<p2
��03min����CH3OH��ʾ�ķ�Ӧ����v(CH3OH)=___(������λС��)��
��200��ʱ���÷�Ӧ��ƽ�ⳣ��K=__��������200��ﵽƽ��ĺ����ܱ��������ټ���2 mol CO��2 mol H2��2 mol CH3OH�������¶Ȳ��䣬��ѧƽ��__(�������������)�ƶ���
(3)�����ͬ�ļס������������У��ֱ��е����ʵ�����SO2��O2������ͬ�¶��·�����Ӧ��2SO2+O2![]() 2SO3�����ﵽƽ�⣬��������У�����������������䣬����������ѹǿ���䣬����������SO2��ת����Ϊp%������������SO2��ת������______________��
2SO3�����ﵽƽ�⣬��������У�����������������䣬����������ѹǿ���䣬����������SO2��ת����Ϊp%������������SO2��ת������______________��
A. ����p% B. ����p% C. С��p% D. ���ж�