��Ŀ����
(8��)ijѧ��Ϊ��̽��п�����ᷴӦ�����е����ʱ仯������100 mLϡ�����м���������п�ۣ�����ˮ�������ռ���Ӧ�ų���������ʵ���¼����(�ۼ�ֵ�����������Ϊ������)��
| ʱ��(min) | 1 | 2 | 3 | 4 | 5 |
| �������(mL) | 50 | 120 | 232 | 290 | 310 |
ԭ���� ��
(2)��2��3����ʱ����������Ũ�ȱ仯����ʾ�ĸ÷�Ӧ����(����Һ�������)
(3)�����Ӧ̫���ң�Ϊ�˼�����Ӧ���ʶ��ֲ����ٲ����������������������зֱ�������
����������Һ������Ϊ���е���__________��
A������ˮ B��NaCl��Һ C��NaNO3��Һ D��CuSO4��Һ E��Na2CO3��Һ
��2-3min ��Ӧ���ȣ��¶����ߣ���Ӧ���ʼӿ�
��0.1mol/(L��min) ��AB
���������������1����0��1��1��2��2��3��3��4��4��5minʱ����У��������������ֱ��ǣ�50 mL�� 70mL ��112mL��68mL��20 mL���ɴ�֪��Ӧ��������ʱ���Ϊ2-3min����Ӧ���ʸտ�ʼ�����������С�뷴Ӧ���Ũ���أ�ֻ�����뷴Ӧ����ЧӦ�йء�
��2����2��3����ʱ����ڣ�n(H2)= 0.112L/22.4L/moL=0.005mol����������������ʵ���=2n(H2)����ԣ�HCl��=0.1mol/(L��min)��
��3��A����������ˮ��H+Ũ�ȼ�С����Ӧ���ʼ�С��������������������ȷ��B������NaCl��Һ��H+Ũ�ȼ�С����Ӧ���ʼ�С��������������������ȷ��C������NaNO3��Һ������NO���壬Ӱ��������������������D������CuSO4��Һ��Zn�û���Cu�γ�ԭ��أ�ʹ��Ӧ��������Ӱ��������������������E������Na2CO3��Һ������H+��ʹH+Ũ�ȼ�С��Ӱ��������������������
���㣺��ѧ��Ӧ���ʵļ��㣬Ӱ�컯ѧ��Ӧ���ʵ����ء�

100mL 6 mol / L H2SO4������п�۷�Ӧ����һ���¶��£�Ϊ�˼�����Ӧ���е����ʣ����ֲ�Ӱ����������������������Ӧ���м���������
| A���������Һ | B������ | C���Ȼ��ƣ����壩 | D������ |
ú��������úΪԭ�ϣ�������ѧ�ӹ�ʹúת��Ϊ���塢Һ�塢����ȼ���Լ����ֻ�����Ʒ�Ĺ�ҵ���̡�
��1����ˮ����ͨ�����ȵ�̿���ɲ���ˮú������ӦΪ��C(s)��H2O(g)  CO(g)��H2(g) ��H����131.3 kJ��mol��1һ���¶��£���һ���ݻ��ɱ���ܱ������У�����������Ӧ���������жϸ÷�Ӧ�ﵽ��ѧƽ��״̬���� (����ĸ����ͬ)
CO(g)��H2(g) ��H����131.3 kJ��mol��1һ���¶��£���һ���ݻ��ɱ���ܱ������У�����������Ӧ���������жϸ÷�Ӧ�ﵽ��ѧƽ��״̬���� (����ĸ����ͬ)
A�������е�ѹǿ����
B��1 mol H��H�����ѵ�ͬʱ����2 mol H��O��
C��v��(CO)��v��(H2O)
D��c(CO)��c(H2)
��2������ͬ����CO(g)��H2O(g)�ֱ�ͨ�뵽���Ϊ2 L�ĺ����ܱ������У����з�ӦCO(g)��H2O(g)  CO2(g)��H2(g)���õ������������ݣ�
CO2(g)��H2(g)���õ������������ݣ�
| ʵ �� �� | �� �� /�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| H2O | CO | H2 | CO | |||
| 1 | 650 | 2 | 4 | 1.6 | 2.4 | 5 |
| 2 | 900 | 1 | 2 | 0.4 | 1.6 | 3 |
| 3 | 900 | a | b | c | d | t |
�ٸ÷�Ӧ���淴ӦΪ (������š�)�ȷ�Ӧ��
����ʵ��3Ҫ�ﵽ��ʵ��2��ͬ��ƽ��״̬(�������ʵ����������ֱ����)����t<3 min����a��bӦ����Ĺ�ϵ�� (�ú�a��b����ѧʽ��ʾ)��
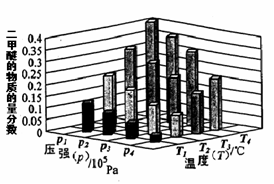
(3)Ŀǰ��ҵ����һ�ַ�������CO2�������״���һ�������·�����Ӧ��CO2(g)+3H2(g)
 CH3OH(g) +H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯�������Ϊ1 L�ĺ����ܱ������У�����1 mol CO2��3 mol H2�� ���д�ʩ����ʹ c(CH3OH)�������
CH3OH(g) +H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯�������Ϊ1 L�ĺ����ܱ������У�����1 mol CO2��3 mol H2�� ���д�ʩ����ʹ c(CH3OH)������� 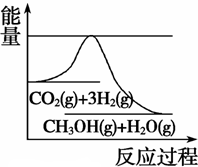
A�������¶�
B������N2(g)��ʹ��ϵѹǿ����
C����H2O(g)����ϵ�з������
D���ٳ���0.5 mol CO2��1.5 mol H2
 CH3OH(g)+H2O(g)
CH3OH(g)+H2O(g)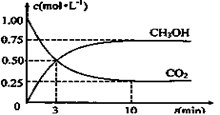
 (NH4)2CO3(aq) ��H1
(NH4)2CO3(aq) ��H1
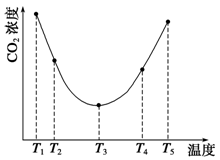
 CH3OCH3(g) + H2O(g)����֪һ�������£��÷�Ӧ��CO��ƽ��ת�������¶ȡ�Ͷ�ϱ�[n(H2) / n(CO)]�ı仯����������ͼ��
CH3OCH3(g) + H2O(g)����֪һ�������£��÷�Ӧ��CO��ƽ��ת�������¶ȡ�Ͷ�ϱ�[n(H2) / n(CO)]�ı仯����������ͼ��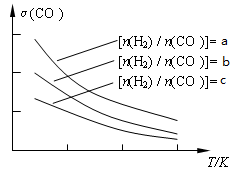
 NaNO3��s��+ClNO��g�� K1 ?H < 0 ��I��
NaNO3��s��+ClNO��g�� K1 ?H < 0 ��I�� xC��g��+2D��g��������5min��Ӧ�ﵽƽ��״̬�������ڵ�ѹǿ��С����֪D��ƽ����Ӧ����Ϊ0.2mol/��L?min��,����д���пհף�
xC��g��+2D��g��������5min��Ӧ�ﵽƽ��״̬�������ڵ�ѹǿ��С����֪D��ƽ����Ӧ����Ϊ0.2mol/��L?min��,����д���пհף�