��Ŀ����
����Ŀ�������������ض�����Ҫ�Ĺ�ҵ��Ʒ����ش��������⣺
��1����ҵұ�����Ļ�ѧ����ʽ��_________________��������Ӧʽ��__________��
������ת�Ƶ���0.6 mol����Al��������________ g��
��2����������������Һ��Ӧ�����ӷ���ʽ��_____________________________��
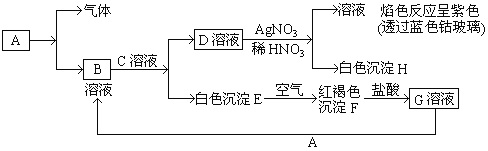
��3����ҵ��Ʒ�������ص���Һ�к���ijЩ����������ӣ��������ӽ���Ĥ������ᴿ��������װ�������ӽ���Ĥ(ֻ����������ͨ��)������ԭ������ͼ��ʾ��
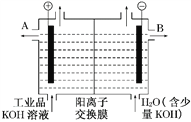
�ٸõ��۵�������Ӧ��__________________��
��ͨ�翪ʼ������������ҺpH������ԭ��Ϊ___________________________
�۳�ȥ���ʺ������������Һ��Һ�����________(�A����B��)������
���𰸡� ![]()
![]() 5.4 2Al+2OH-+6H2O=2[Al(OH)4]-+3H2O
5.4 2Al+2OH-+6H2O=2[Al(OH)4]-+3H2O ![]()
![]() B
B
�������������������1����ҵ�ϵ�����������������������������õ��ӷ�����ԭ��Ӧ������������Ӧʽ��������������������2����������������Һ��Ӧ�������ǻ�������غ���������3�����������������Һ��ʵ�ʵĵ��ˮ����������������ʧ�������������������������ӵõ�������������������Ӧʽ��![]() ��������������Ӧʽ
��������������Ӧʽ![]() �������������������غ�������
�������������������غ�������
��������1����ҵ�ϵ��������������������������Ӧ����ʽ��![]() ��������Ӧʽ
��������Ӧʽ![]() ������������Ӧʽ��ת�Ƶ���0.6 mol����0.2molAl��������0.2mol
������������Ӧʽ��ת�Ƶ���0.6 mol����0.2molAl��������0.2mol![]() 27g/mol=5.4g����2����������������Һ��Ӧ�������ǻ�������غ���������Ӧ���ӷ���ʽ��2Al+2OH-+6H2O=2[Al(OH)4]-+3H2O����3���ٵ������������Һ����������������ʧ��������������������Ӧʽ��
27g/mol=5.4g����2����������������Һ��Ӧ�������ǻ�������غ���������Ӧ���ӷ���ʽ��2Al+2OH-+6H2O=2[Al(OH)4]-+3H2O����3���ٵ������������Һ����������������ʧ��������������������Ӧʽ��![]() �������������ӵõ�������������������Ӧʽ��
�������������ӵõ�������������������Ӧʽ��![]() ��������Ũ�Ƚ���������������Ũ����������������������ҺpH��������������������Ӧʽ��
��������Ũ�Ƚ���������������Ũ����������������������ҺpH��������������������Ӧʽ��![]() ������������������������������������Һ��Һ�����B������
������������������������������������Һ��Һ�����B������

����Ŀ�����в���Ԫ�ص�ԭ�ӽṹ�ص������
X | L���������K���������3�� |
Y | ������Ӳ�������ԭ������ |
Z | L���������K���M�������֮�� |
W | �����������Ǵ�����������2.5�� |
��1������Wԭ�ӽṹʾ��ͼ________________________��
��2��Ԫ��X��Ԫ��Z��ȣ��ǽ����Խ�ǿ����________(��Ԫ������)��д��һ���ܱ�ʾX��Z�ǽ�����ǿ����ϵ�Ļ�ѧ��Ӧ����ʽ��________________________________________��
��3��X��Y��Z��W����Ԫ���γɵ�һ�����ӻ������ˮ��Һ��ǿ���ԣ��û�����Ļ�ѧʽΪ____________________��
��4��Ԫ��X��Ԫ��Y��ԭ�Ӹ�����1��1�����γɵĻ�����Q��д��Q�ĵ���ʽ________��Ԫ��W��Ԫ��Y�����γɵĻ�����M��Q��M�ĵ���������ȡ���MΪȼ�ϣ�QΪ����������������ƽ����������������ġ�������Ȼ�����ȶ����ڵ����ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ��____________________________________��