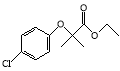
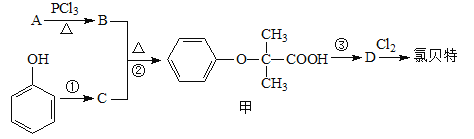
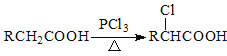
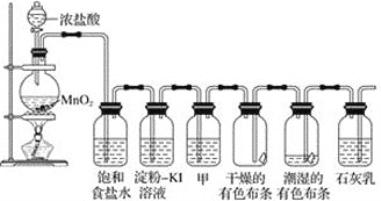
��Ŀ����
����Ŀ����ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��1����Ũ������HCl�����ʵ���Ũ��Ϊ______molL-1��
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����______��
A����Һ��HCl�����ʵ��� B����Һ��Ũ�� C����Һ��Cl-����Ŀ D����Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����480mL���ʵ���Ũ��Ϊ0.400molL-1��ϡ���ᡣ
�ٸ�ѧ����Ҫ��ȡ______mL����Ũ����������ơ�
�������ƹ����У�����ʵ������������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿������������A��ʾ��ƫ����B��ʾ��ƫС������C��ʾ����Ӱ�족����
a������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ��______��
b�����ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ______��
��4�������ͬѧ�ɹ�������0.400molL-1�����ᣬ�����ø������кͺ�0.4g NaOH��NaOH��Һ�����ͬѧ��ȡ______mL���ᡣ
���𰸡�11.9BD16.8BB25.00(25Ҳ�ɸ���)
��������
��1������c=![]() ���㣻��2��Ħ����������ǿ�����ʣ��������仯����Һ�Ǿ��ȵģ���Һ���ܶȡ�Ũ�Ȳ�������仯����3����ʵ����û��480mL����ƿ��Ӧѡ��500mL����ƿ������ϡ�Ͷ��ɣ�ϡ��ǰ��HCl�����ʵ������䣬�ݴ˼�����ҪŨ�����������ڷ����������������ʵ�������Һ�����Ӱ�죬����c=
���㣻��2��Ħ����������ǿ�����ʣ��������仯����Һ�Ǿ��ȵģ���Һ���ܶȡ�Ũ�Ȳ�������仯����3����ʵ����û��480mL����ƿ��Ӧѡ��500mL����ƿ������ϡ�Ͷ��ɣ�ϡ��ǰ��HCl�����ʵ������䣬�ݴ˼�����ҪŨ�����������ڷ����������������ʵ�������Һ�����Ӱ�죬����c=![]() �ж϶�������ҺŨ�ȵ�Ӱ����
�ж϶�������ҺŨ�ȵ�Ӱ����
��1����Ũ������HCl�����ʵ���Ũ��C=![]() =11.9mol/L����2��A����Һ��HCl�����ʵ�����Сȡ������Һ�����С����ҺŨ�ȣ�ѡ��A����B����Һ���о�һ�ԣ���Һ��Ũ������Һ�����С�أ�ѡ��B��ȷ��C����Һ��Cl-����Ŀ����ҺŨ�ȡ���Һ�����С�����ʻ�ѧʽ����йأ�ѡ��C����D����Һ���о�һ�ԣ���Һ���ܶ�����Һ�����С�أ�ѡ��D��ȷ����ѡBD����3��������ҪŨ�������ΪV����������Һϡ���������ʵ����ʵ������ֲ����V��11.9mol/L=0.400molL-1��500mL�����V=16.8mL����a������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�棬������ȡ��Ũ�������ƫС�������Ȼ�������ʵ���ƫС����ҺŨ��ƫС��b�����ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ��������Һ���ƫ����ҺŨ��ƫС����4�����ɹ�ϵʽ��HCl��NaOH��֪��n��HCl��=n��NaOH��=
=11.9mol/L����2��A����Һ��HCl�����ʵ�����Сȡ������Һ�����С����ҺŨ�ȣ�ѡ��A����B����Һ���о�һ�ԣ���Һ��Ũ������Һ�����С�أ�ѡ��B��ȷ��C����Һ��Cl-����Ŀ����ҺŨ�ȡ���Һ�����С�����ʻ�ѧʽ����йأ�ѡ��C����D����Һ���о�һ�ԣ���Һ���ܶ�����Һ�����С�أ�ѡ��D��ȷ����ѡBD����3��������ҪŨ�������ΪV����������Һϡ���������ʵ����ʵ������ֲ����V��11.9mol/L=0.400molL-1��500mL�����V=16.8mL����a������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�棬������ȡ��Ũ�������ƫС�������Ȼ�������ʵ���ƫС����ҺŨ��ƫС��b�����ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ��������Һ���ƫ����ҺŨ��ƫС����4�����ɹ�ϵʽ��HCl��NaOH��֪��n��HCl��=n��NaOH��=![]() =0.01mol��V��HCl��=
=0.01mol��V��HCl��=![]() =0.025L=25mL��
=0.025L=25mL��