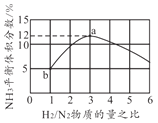
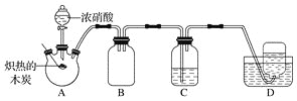
��Ŀ����
����Ŀ����NAΪ�����ӵ�������ֵ������˵������ȷ���ǣ� ��
A.һ�������£��ϳɰ���Ӧ����0.5molN2������Ӧ����Ӧ��ת�Ƶ�����Ϊ3NA
B.10�˻�������2-�����Ļ��Һ�У�̼Ԫ�ص���������Ϊ72%����������������ԭ����ĿΪ![]() NA
NA
C.����ͭ�뺬0.4molHNO3��Ũ���ᷴӦ����Ӧ�е���ת����Ϊ0.2NA
D.���³�ѹ����28g�����辧�壬������������Si��Si����ĿΪ2NA
���𰸡�C
��������
A����Ӧ��N2ת��ΪNH3����Ԫ����0�۱�Ϊ-3�ۣ���1mol N2���뷴Ӧת��6mol���ӣ���0.5molN2������Ӧ����Ӧ��ת�Ƶ�����Ϊ3mol���ӣ�����Ϊ3 NA����A��ȷ��
B��������ķ���ʽΪC6H12��2-�����ķ���ʽΪC3H8O���൱��C3H6��H2O��̼Ԫ�ص���������Ϊ72%������![]() �����10�˻��Һ�к�CH2������Ϊ8.4g������H2O��Ϊ1.6g�������к��е���ԭ����ĿΪ
�����10�˻��Һ�к�CH2������Ϊ8.4g������H2O��Ϊ1.6g�������к��е���ԭ����ĿΪ![]() NA=
NA=![]() NA����B��ȷ��
NA����B��ȷ��
C������ͭ�뺬0.4 mol HNO3��Ũ���ᷴӦ����ֻ����NO2��������ӦΪCu+4HNO3(Ũ)= Cu(NO3)2+2H2O+2NO2������Ԫ����+5�۱�Ϊ+4�ۣ�0.4molŨHNO3������뷴Ӧ������0.2mol����ԭ������ת����ĿΪ0.2NA�������ŷ�Ӧ������Ũ�����ϡ������������NO2��ΪNO����Ԫ����+5�۱�Ϊ+2�ۣ�ת�Ƶ�����Ŀ���࣬�����ת����ĿΪ����0.2 NA����C����
D����������һ��Siԭ��ƽ����������Si��Si�������³�ѹ����28g�����辧��Ϊ1mol��������������Si��Si����ĿΪ2NA����D��ȷ��
��ѡC��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�