��Ŀ����
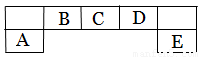
 ����������Ԫ��A��B��C��D��E��Ԫ�����ڱ��е�λ������ͼ��ʾ������AΪ�ؿ��к�����ߵĽ���Ԫ�ء�
����������Ԫ��A��B��C��D��E��Ԫ�����ڱ��е�λ������ͼ��ʾ������AΪ�ؿ��к�����ߵĽ���Ԫ�ء�
���û�ѧ����ش��������⣺
��1��DԪ�������ڱ��е�λ�ã�???????????????
��2��A��D ��EԪ�ؼ����Ӱ뾶�ɴ�С��˳��Ϊ_____��______ ��______ (�������� )
 ��3��F��Dͬ���������ڣ�����̬�⻯���ȶ��ԵĴ�С??????? ��???????? ���������ţ�
��3��F��Dͬ���������ڣ�����̬�⻯���ȶ��ԵĴ�С??????? ��???????? ���������ţ�
��4���ø����������京��10���ӵ�DԪ���⻯�����ʱ��һ���������ͷ�һ�����ӣ�ͬʱ����һ�־��нϸ������Ե������ӣ���д���������ӵĵ���ʽ????? ??????? �����������д��ڵĻ�ѧ����??????????????????? ��
 ��5��CԪ�صļ��⻯����EԪ�ص�����������ˮ���ﷴӦ�����ɻ�����K����K��ˮ��Һ��_____�ԣ����������������������������������������ӷ���ʽ��ʾ��ԭ��???????????????? ��
��5��CԪ�صļ��⻯����EԪ�ص�����������ˮ���ﷴӦ�����ɻ�����K����K��ˮ��Һ��_____�ԣ����������������������������������������ӷ���ʽ��ʾ��ԭ��???????????????? ��
��6��������AC�����Ժã�������ϵ��С�������õ����ȳ�����ϡ������Ʊ�AC��һ�ַ���Ϊ����AԪ�����������̿��C�ĵ�����1600 ~ 1750������AC��ÿ����1 mol AC������18 g̼������b kJ������������������Ϊ25����101��3 kPa�����£�д���÷�Ӧ���Ȼ�ѧ����ʽ????????????????????????????? ��
��7����Fe��Cu�Ļ�����м���һ����C������������ˮ����ϡ��Һ����ַ�Ӧ��ʣ�����m1 g���������м���ϡ���ᣬ��ַ�Ӧ����ʣ�� m2 g ������˵����ȷ����?????????? ��
a������ϡ����ǰ�ͼ���ϡ��������Һ�п϶�����Cu2+?
b������ϡ����ǰ�ͼ���ϡ��������Һ�п϶�����Fe2+?
c��m1 һ������m2
d��ʣ�����m1 g ��һ���е���ͭ��ʣ�����m2 g ��һ��û�е���ͭ
��1���ڶ����ڵ���A�壨1�֣�
��2��Cl-> O2-> Al3+��2�֣�
��3��H2O > H2S��1�֣�
��4��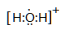 ? ��1�֣�??? ���Թ��ۼ���1�֣�
? ��1�֣�??? ���Թ��ۼ���1�֣�
��5�����ԣ�1����?? NH4+ + H2O? 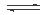 ? NH3��H2O + H+��2����
? NH3��H2O + H+��2����
 ��6��3C(s) + Al2O3(s) + N2 (g) = 2AlN(s) + 3CO(g)? ��H= +2b kJ/mol��3����
��6��3C(s) + Al2O3(s) + N2 (g) = 2AlN(s) + 3CO(g)? ��H= +2b kJ/mol��3����
��7��b��c��2����
��������
�����������������֪��AΪ�ؿ��к�����ߵĽ���Ԫ�أ���AΪ��Ԫ�أ���ϳ���Ԫ����Ԫ�����ڱ��е�λ���жϣ�BΪ̼Ԫ�أ�CΪ��Ԫ�أ�DΪ��Ԫ�أ�EΪ��Ԫ�ء���1��DΪ��Ԫ�أ������ڱ��е�λ�ã��ڶ����ڵ���A�壻��2��AΪ��Ԫ�أ�DΪ��Ԫ�أ�EΪ��Ԫ�أ��������뾶�Ƚ�ԭ���жϣ������Ӱ뾶�ɴ�С��˳��ΪCl-> O2-> Al3+�� ��3��F����ͬ���������ڣ���FΪ��Ԫ�أ�ͬ�������ϵ�����̬�⻯����ȶ�������������̬�⻯���ȶ��ԵĴ�СH2O > H2S����4������10���ӵ�DԪ���⻯�����Ϊˮ���ӣ��ø�����������ˮ����ʱ��һ���������ͷ�һ�����ӣ�ͬʱ����һ�־��нϸ������Ե������ӣ��������ӵĵ���ʽ���𰸣����������д��ڵĻ�ѧ���м��Թ��ۼ���
��3��F����ͬ���������ڣ���FΪ��Ԫ�أ�ͬ�������ϵ�����̬�⻯����ȶ�������������̬�⻯���ȶ��ԵĴ�СH2O > H2S����4������10���ӵ�DԪ���⻯�����Ϊˮ���ӣ��ø�����������ˮ����ʱ��һ���������ͷ�һ�����ӣ�ͬʱ����һ�־��нϸ������Ե������ӣ��������ӵĵ���ʽ���𰸣����������д��ڵĻ�ѧ���м��Թ��ۼ��� ��5����Ԫ�صļ��⻯������Ԫ�ص�����������ˮ���ﷴӦ�����ɻ������Ȼ�泥�ˮ��Һ�����ԣ������ӷ���ʽ��ʾ��ԭ��NH4+ + H2O?
��5����Ԫ�صļ��⻯������Ԫ�ص�����������ˮ���ﷴӦ�����ɻ������Ȼ�泥�ˮ��Һ�����ԣ������ӷ���ʽ��ʾ��ԭ��NH4+ + H2O? 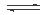 ? NH3��H2O + H+����6����������֪������������̿�͵�����1600 ~ 1750�����ɵ�������ÿ����1 mol ������������18 g̼������b kJ������������������Ϊ25����101��3 kPa�����£��÷�Ӧ���Ȼ�ѧ����ʽΪ3C(s) + Al2O3(s) + N2 (g) = 2AlN(s) + 3CO(g)? ��H= +2b kJ/mol����7����Fe��Cu�Ļ�����м���һ��������ϡ��Һ���������Ļ�ԭ�Ա�ͭǿ�����������ᷴӦ����ַ�Ӧ����������������������������ͭ��ʣ�����Ϊͭ������ͭ�Ļ���m1 g���������м���ϡ���ᣬ����3Cu+2NO3-+8H+=3Cu2++2NO��+4H2O����3Fe+2NO3-+8H+=3Fe2++2NO��+4H2O��a������ϡ����ǰ�ͼ���ϡ��������Һ�ж���һ����Cu2+������b������ϡ����ǰ�ͼ���ϡ��������Һ�п϶�����Fe2+����ȷ��c��m1 һ������m2����ȷ��d��ʣ�����m1 g ��һ���е���ͭ��ʣ�����m2 g �п����е���ͭ������ѡbc��
? NH3��H2O + H+����6����������֪������������̿�͵�����1600 ~ 1750�����ɵ�������ÿ����1 mol ������������18 g̼������b kJ������������������Ϊ25����101��3 kPa�����£��÷�Ӧ���Ȼ�ѧ����ʽΪ3C(s) + Al2O3(s) + N2 (g) = 2AlN(s) + 3CO(g)? ��H= +2b kJ/mol����7����Fe��Cu�Ļ�����м���һ��������ϡ��Һ���������Ļ�ԭ�Ա�ͭǿ�����������ᷴӦ����ַ�Ӧ����������������������������ͭ��ʣ�����Ϊͭ������ͭ�Ļ���m1 g���������м���ϡ���ᣬ����3Cu+2NO3-+8H+=3Cu2++2NO��+4H2O����3Fe+2NO3-+8H+=3Fe2++2NO��+4H2O��a������ϡ����ǰ�ͼ���ϡ��������Һ�ж���һ����Cu2+������b������ϡ����ǰ�ͼ���ϡ��������Һ�п϶�����Fe2+����ȷ��c��m1 һ������m2����ȷ��d��ʣ�����m1 g ��һ���е���ͭ��ʣ�����m2 g �п����е���ͭ������ѡbc��
���㣺����Ԫ���ƶϡ�Ԫ�������ɼ�������ʵĽṹ�����ʣ��漰����ʽ����д������ˮ�⡢�Ȼ�ѧ����ʽ�������ǿ�����ԡ�

| A��ԭ�Ӱ뾶��A��D��C��B | B��B��C��D�ֱ���A�γɵĻ�����һ��������ͬ�Ļ�ѧ�� | C������������Ӧˮ��������ԣ�D��C | D�������£�����B�ܴ�������Ũ������ |
| A��ԭ�ڰ뾶A��B��C | B��A����̬�⻯���ȶ��Դ���C����̬�⻯���ȶ��� | C��A��C��Ԫ����������������ˮ���ϵõ���Ӧ���� | D������ʱ��A���ʿ��Դ�C�����������û��õ�C���� |
| A��ԭ�Ӱ뾶��С�����˳��r��C����r��D����r��E�� | B��Ԫ��D��E�ֱ���A�γɵĻ���������ȶ��ԣ�E��D | C��Ԫ��D������������Ӧˮ��������Ա�E��ǿ | D��Ԫ��B�ֱ���A��C�γɵĻ������л�ѧ����������ȫ��ͬ |
 ����������Ԫ��A��B��C��D��E��ԭ������������������ԭ�Ӻ���ĵ��Ӳ���֮��Ϊ10��BԪ�صĻ���������࣬��Ŀ�Ӵ�C��D����Ԫ���γɵĵ����ǿ����к����������ʣ�D��E��Ԫ�ؿ����������ֲ�ͬ�����ӻ����
����������Ԫ��A��B��C��D��E��ԭ������������������ԭ�Ӻ���ĵ��Ӳ���֮��Ϊ10��BԪ�صĻ���������࣬��Ŀ�Ӵ�C��D����Ԫ���γɵĵ����ǿ����к����������ʣ�D��E��Ԫ�ؿ����������ֲ�ͬ�����ӻ���� NH3?H2O+H+
NH3?H2O+H+ 2NO2
2NO2