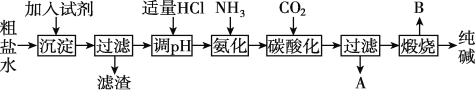
��Ŀ����
����Ŀ������Ҫ��ش��������⣺
��1����Ԫ����һ�ֳ����ķǽ���Ԫ�أ���ԭ�ӵ�ԭ�ӽṹʾ��ͼΪ_______,��Ȼ���д��ڵ�ij�ֵ����˸�ԭ�ӽ�ϣ������ʽΪ_______,���ҹ��Ŵ������ʳ������������ڻ�ҩ����ըʱ���������һ�ִ̼�����ζ�����壬�������������ԭ���ǣ���ѧ����ʽ��_______________________�����ʻ��ܹ������Ԫ�ط�Ӧ����д�����������ͭ�ķ�Ӧ����ʽΪ______________________,�����������ӵĵ���ʽΪ_________��
��2��Ũ������һ����Ҫ�Ļ���ԭ�ϣ�����____�ԡ�_____�ԡ�____�ԣ���д��ͭ��Ũ���ᷴӦ�ķ���ʽ_________________,����Ũ����Ҳ�ܹ���ijЩ�ǽ���Ԫ�ص��ʷ�Ӧ����д����̿��Ũ���ᷴӦ�Ļ�ѧ����ʽ__________________��
��3����Ԫ���Ǵ����к�����ߵ�Ԫ�أ��䵥�ʵĵ���ʽ___________�����ڸõ��ʱȽ��ȶ���ֻ���벿�ֻ��ý�����Ӧ�������ڵ�ȼ�����£��������þ��Ӧ����д���÷�Ӧ����ʽ___________________________����ҵ�̵��Ļ�ѧ����ʽΪ��________________________________��
���𰸡� 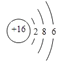 S8
S8 ![]()
![]()
![]() ǿ���� ��ˮ ��ˮ����ѻӷ����ȣ���ѡ��������
ǿ���� ��ˮ ��ˮ����ѻӷ����ȣ���ѡ�������� ![]()
![]()
![]()
![]()
![]() ��ע������������
��ע������������
�����������������������Ҫ����ԭ�ӽṹʾ��ͼ������ʽ�����ֻ�����ѧ����ʽ��д������ʽ��Ũ������������ԣ��DZȽϻ��������͡�
��������1����Ϊ16��Ԫ�أ�ԭ�ӽṹʾ��ͼ��![]() ��8��ԭ�ӽ�ϵķ����е�Ȼ����8��Sԭ�ӣ�����ʽ��S8���ڻ�ҩ��Ҫ�����S��C��KNO3����ըʱ�����Ĵ̼�����ζ������SO2����Ӧ����ʽΪS+2KNO3+3C=K2S+3CO2��+N2����S��Cu��Ӧ����Cu2S����Ӧԭ����2Cu+S
��8��ԭ�ӽ�ϵķ����е�Ȼ����8��Sԭ�ӣ�����ʽ��S8���ڻ�ҩ��Ҫ�����S��C��KNO3����ըʱ�����Ĵ̼�����ζ������SO2����Ӧ����ʽΪS+2KNO3+3C=K2S+3CO2��+N2����S��Cu��Ӧ����Cu2S����Ӧԭ����2Cu+S![]() Cu2S����������������S2-������ʽ��
Cu2S����������������S2-������ʽ��![]() ����ȷ�𰸣�
����ȷ�𰸣�![]() ��S8��S+KNO3+3C=K2S+3CO2��+N2����2Cu+S
��S8��S+KNO3+3C=K2S+3CO2��+N2����2Cu+S![]() Cu2S��
Cu2S��![]() ��(2)Ũ�������Ҫ���ʰ�����ˮ�ԡ���ˮ�ԡ�ǿ�����ԡ����ԡ��ѻӷ��Ե���Cu��Ũ������ȷ�����Ӧ��Cu+2H2SO4
��(2)Ũ�������Ҫ���ʰ�����ˮ�ԡ���ˮ�ԡ�ǿ�����ԡ����ԡ��ѻӷ��Ե���Cu��Ũ������ȷ�����Ӧ��Cu+2H2SO4![]() CuSO4+SO2��+2H2O��C��Ũ���ᷴӦ��C+2H2SO4
CuSO4+SO2��+2H2O��C��Ũ���ᷴӦ��C+2H2SO4![]() CO2��+2SO2��+2H2O����ȷ�𰸣���ˮ����ˮ��ǿ������Cu+2H2SO4
CO2��+2SO2��+2H2O����ȷ�𰸣���ˮ����ˮ��ǿ������Cu+2H2SO4![]() CuSO4+SO2��+2H2O��C+2H2SO4
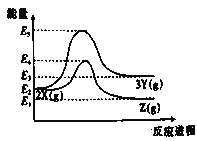
CuSO4+SO2��+2H2O��C+2H2SO4![]() CO2��+2SO2��+2H2O����3��������˫ԭ�ӷ��ӣ�����ʽΪ
CO2��+2SO2��+2H2O����3��������˫ԭ�ӷ��ӣ�����ʽΪ![]() ��������þ��Ӧ���ɵ���þ����Ӧԭ����3Mg+N2
��������þ��Ӧ���ɵ���þ����Ӧԭ����3Mg+N2![]() Mg3N2����ҵ�̵�������N2��H2�ϳ�NH3����Ӧԭ��ΪN2+3H2
Mg3N2����ҵ�̵�������N2��H2�ϳ�NH3����Ӧԭ��ΪN2+3H2![]() 2NH3����ȷ�𰸣�
2NH3����ȷ�𰸣�![]() ��3Mg+N2
��3Mg+N2![]() Mg3N2��N2+3H2
Mg3N2��N2+3H2![]() 2NH3��
2NH3��

 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�����Ŀ������A��D����ͬ��Ԫ�أ�������ѧ��ѧ�г��������ʣ�����֮��ɷ�����ͼ��ʾ�ķ�Ӧ����A��D����������ʾ�����ȥ����
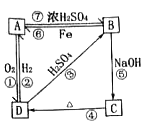
��1��д����Ӧ���ʵ����
���� | B | C | D |
��� | ___ | __ | __ |
��2��������Ӧ�ٵ����У��������ӷ�Ӧ����___����
��3����D��Aת���Ļ�ѧ����ʽ��__��
��B��Cת�������ӷ���ʽ��__��