��Ŀ����
����Ŀ����ˮ�Ǿ����Դ���⣬�Ӻ�ˮ����ȡʳ�κ���Ĺ������£�
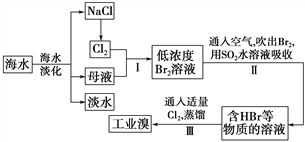
��1�����оٺ�ˮ���������ַ�����________��________��
��2����������ѻ��Br2�����������ֽ�Br2��ԭΪBr������Ŀ����_________��
��3����������SO2ˮ��Һ����Br2�������ʿɴ�95%���йط�Ӧ�����ӷ���ʽΪ_______���ɴ˷�Ӧ��֪�������������⣬�ڹ�ҵ������Ӧ�������Ҫ������_______��
��4��ij��ѧ�о���ѧϰС��Ϊ���˽�ӹ�ҵ�����ᴿ��ķ������������й�����֪��Br2�ķе�Ϊ59 ��������ˮ���ж�����ǿ��ʴ�ԡ����Dzι��������̺�������װ�ü�ͼ��
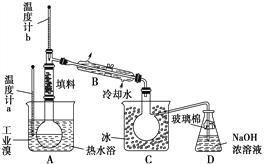
�������������ۣ�
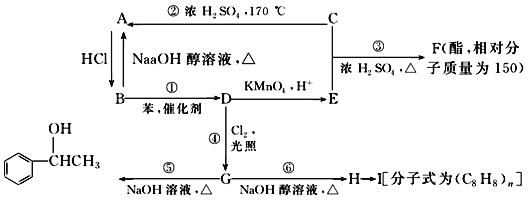
��ͼ������B��������____________��
������ʵ��װ�����������Ӿ��������������ܣ���ԭ����__________��
��ʵ��װ�����������ã�Ҫ�ﵽ�ᴿ���Ŀ�ģ���������ο��ƹؼ�������___________��
��C��Һ����ɫΪ________________��Ϊ��ȥ�ò������Բ���������Cl2���������м���NaBr��Һ����ַ�Ӧ���ٽ��еķ��������________________��
���𰸡���1�������������������ӽ��������е��������֣�
��2��������Ԫ�أ�
��3��SO2+Br2+2H2O=4H++SO42-+2Br-��
��4���������ܣ��������ǿ��ʴ�ԣ����Ը�ʴ����Ҫ�����¶ȼ�b���¶ȣ����ռ�59��ʱ����֣��������ɫ����ȡ����Һ
��������(1)�Ӻ�ˮ��ȡ��ˮ��������������������ˮ������ܼ���ȡ���ͱ���������ȥ��ˮ�е��η֣��е��������ӽ�������ѹ�������ʴ�Ϊ�������������������ӽ��������е�һ�֣�
(2)��������ѻ��Br2����������ֽ�Br2��ԭΪBr-��Ŀ���ǵ�Ũ�ȵ�Br2��Һ����ȡʱ���Ĺ����ԭ�Ϻ���Դ��ת��ΪHBr���ױ���������������Ϊ�嵥�ʣ����ڸ�����Ԫ�أ��ʴ�Ϊ��������Ԫ�أ�
(3)�������������嵥�ʷ�����Ӧ��SO2+Br2+2H2O=H2SO4+2HBr�����ӷ���ʽΪSO2+Br2+2H2O�T4H++2Br-+SO42-������������Ҫ�Ǽ���SO2�������ĺ��ŷţ���ҵ������Ӧ�������Ҫ�����Ƿ�Ӧ����������ǿ�ᣬ��ʴ�豸���ʴ�Ϊ��SO2+Br2+2H2O�T4H++2Br-+SO42-��ǿ����豸�����ظ�ʴ��
(4)����װ��ͼ��֪���ᴿ�����õ�ԭ������������BΪ�����ܣ��ʴ�Ϊ�������ܣ�
�ڿ����¶�59�棬ʹ�嵥�ʻӷ���ͨ�������õ��������ɫ��Һ̬�嵥�ʣ�ͬʱ������Ⱦ������������β�����գ������嵥����һ��ǿ����������ʴ����Ʒ����������װ�ò������������ܣ��ʴ�Ϊ��Br2��ʴ��
�۴ﵽ�ᴿ���Ŀ�ģ�������Ӧ���ƵĹؼ������ǰ��¶ȿ�������ķе�59��C�����ռ����¶��µ���֣��ʴ�Ϊ�������¶ȼ�b���¶ȣ����ռ�59��ʱ����֣�
�ܸò������Բ���������Cl2������NaBr��Һ������Ӧ��2NaBr+Cl2=2NaCl+Br2�����Գ�ȥ���������ɵ��嵥�ʿ���������ķ����õ����ʴ�Ϊ�������ɫ������

 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д� Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�