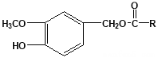
��Ŀ����
����Ŀ����֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A<B<C<D<E������A��B��C��ͬһ���ڵķǽ���Ԫ�ء�������DCΪ���ӻ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ�Ǽ��Է���,�Dz�������ЧӦ����Ҫ���塣B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߡ�����ȱEԪ�ػ�����Dz���D��Eλ��ͬ���塣���������������ش��������⣺(����ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ)
(1)A��B��C�ǽ�������ǿ������˳��Ϊ________��
(2)B���⻯��ķ���ʽ��________��B���⻯������ˮ�ĵ��뷽��ʽΪ
________________________________________________________________________��
(3)д��������AC2�ĵ���ʽ��________������________(����ԡ��Ǽ��ԡ�)���γɵķǼ��Է��ӡ�
(4)B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽ��_________________________________________________��
���𰸡� O>N>C NH3 NH3+H2O![]() NH3��H2O
NH3��H2O![]() NH4++OH��
NH4++OH�� ![]() ���� 4Mg+10HNO3��ϡ��=4Mg(NO3)2+NH4NO3+3H2O
���� 4Mg+10HNO3��ϡ��=4Mg(NO3)2+NH4NO3+3H2O
��������������DCΪ���ӻ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��˵��D��C����һ���ڣ�DӦΪMgԪ�أ�AC2Ϊ�Ǽ��Է��ӣ��Dz�������ЧӦ����Ҫ���壬������ӦΪCO2����AΪCԪ�أ�CΪOԪ�أ���BΪNԪ�أ�����ȱEԪ�ػ�����Dz���D��Eλ��ͬ���壬��EΪCaԪ�ء�
(1)ͬһ���ڣ������ң�Ԫ�صķǽ���������ǿ���ǽ�������ǿ������˳��ΪO>N>C���ʴ�Ϊ��O>N>C��
(2)BΪNԪ�أ���Ӧ���⻯��ΪNH3����������ˮ�����������һˮ�ϰ���һˮ�ϰ��ĵ��뷽��ʽΪ��NH3+H2ONH3H2ONH4++OH-���ʴ�Ϊ��NH3��NH3+H2ONH3H2ONH4++OH-��
(3)AC2ΪCO2��Ϊ���ۻ��������ʽΪ![]() ��������̼Ϊֱ���ͽṹ�����ɼ��Լ��γɵķǼ��Է��ӣ��ʴ�Ϊ��
��������̼Ϊֱ���ͽṹ�����ɼ��Լ��γɵķǼ��Է��ӣ��ʴ�Ϊ��![]() �����ԣ�
�����ԣ�
(4)N����ͼ�Ϊ-3��DΪMgԪ�أ���Ӧ����NH4NO3����Ӧ�ķ���ʽΪ��4Mg+10HNO3=4Mg(NO3)2+NH4NO3 +3H2O���ʴ�Ϊ��4Mg+10HNO3=4Mg(NO3)2+NH4NO3 +3H2O��