��Ŀ����
18���ҹ���������Ԥ����������Ҫ�����������棺�������������NOx������������������ִ�����Ⱦ���Ũ�ȣ���1�����뵪�������йص�ȫ��������Դ�������������ac������ĸ��ţ���
a�����ꡡ��b��ɳ��������c���⻯ѧ���� d������ЧӦ
��������������ø�ѹ��������������˵�Ӿ����
��2��Ϊ�˽�������β���Դ�������Ⱦ��Ŀǰ����Ч�ķ����Ǹ�������װβ������װ�ã����ܽ�β���е�һ����̼��NO�ڴ��������£�������Ӧת��Ϊ�����壬�䷴Ӧ�Ļ�ѧ����ʽΪ2NO+2CO$\frac{\underline{\;����\;}}{\;}$N2+2CO2��
��3����д��ʵ�������ɹ���������ȡ�����Ļ�ѧ����ʽ2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
�����ռ�һƿ����İ�����ѡ��ͼ1�е�װ�ã�������˳��Ϊ������װ�á�d��c��f��e��i��������������Сд��ĸ��ʾ��

��ʵ����Ҳ����ͼ2��ʾװ����ȡ��������ƿ�ڹ����ѡ��ab��ѡ��ѡ��Ĵ��ţ���
a����ʯ�� b����ʯ�� c���������� d������������
�ܴ��������£�NH3Ҳ����������NOx����Ⱦ���������ֶԻ����������ʣ���д��NH3��NO2��Ӧ�Ļ�ѧ����ʽΪ6NO2+8NH3$\frac{\underline{\;����\;}}{\;}$7N2+12H2O���÷�Ӧ��ÿ����0.5molN2ת��1.7mol���ӣ�
���� ��1����a�����꣺��Ҫ�ǹ�ҵ�ŷŵķ�����������͵�����������������ڻҳ�������������Ӧ��������������ˮ�������������������ˮ�������ᣮʹ��ˮPH��5.6��
b�����������������������ľ�������˶������Ĺ̶���ɳ���Ƚ϶࣬����ȼ�ջ�ʯȼ��Ҳ����������Ŀ�����Ⱦ������Ҳ�����ɳ����������
c���⻯ѧ��������Ҫ������β���еĵ�����������̼�⻯�����������������·���һϵ�з�Ӧ����������ɫ��������Σ��������
d����ɫ��Ⱦ�����Ƕ��ѽ����������������ָ���ϴ�����Ⱦ���������һ�������ν��
�ڽ�����е�Ӿ�����ʣ�
��2��NO��CO�ڴ���������ת��Ϊ�����Ͷ�����̼��
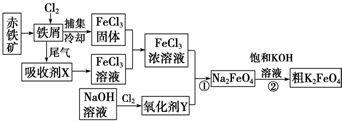
��3����ʵ�����ù����Ȼ�狀����������Ʒ�Ӧ�Ʊ�������
�������Ʊ�һ��˳��Ϊ������װ�ã�����װ�ã��ռ�װ�ã�β������װ�ã���ϰ���Ϊ�������壬�ܶ�С�ڿ����ܶȣ���������ˮ�����ʽ��
��װ��2�е���ƿ���ǹ��壬��ˮ��������ܽ����ʹ�����ų���
��NH3����������NO����Ⱦ������2�ֶԻ����������ʣ�Ӧ���ɵ�����ˮ�����ݻ��ϼ۵ı仯���㣮
��� �⣺��1����a��������������ˮ�������ᣮʹ��ˮpH��5.6����aѡ��
b��ɳ��������������������йأ��뵪�������أ���b��ѡ��
c���⻯ѧ����������β���еĵ����������йأ���cѡ��
d����ɫ��Ⱦ�������йأ���d��ѡ��
ͨ�����Ϸ���֪���뵪�������йصĻ�����Ⱦ�����ꡢ�⻯ѧ������
�ʴ�Ϊ��ac��
�ڽ�����е�Ӿ�����ʣ�����ӵ�Դ�����£�����Ľ������ܹ����������ƶ����������ø�ѹ������������ڼ���������Ԥ��������Ⱦ�������ý����Ӿ�����ʣ�����������������ø�ѹ������������˵�Ӿ����
�ʴ�Ϊ����Ӿ��
��2��NO��CO�ڴ���������ת��Ϊ�����Ͷ�����̼����Ӧ�ķ���ʽΪ��2NO+2CO$\frac{\underline{\;����\;}}{\;}$N2+2CO2��
�ʴ�Ϊ��2NO+2CO$\frac{\underline{\;����\;}}{\;}$N2+2CO2��
��3����ʵ�������ɹ���������ȡ����Ϊ�����Ȼ�狀����������Ʒ�Ӧ����Ӧ�ķ���ʽΪ��Ca��OH��2+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��Ca��OH��2+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��ʵ�����ü��ȹ����Ȼ�狀��������Ƶķ����Ʊ��������Ʊ��������к���ˮ����������Ϊ�������壬Ӧѡ��ʢ�м�ʯ�Ҹ���ܸ������壬������������ˮ���ܶ�С�ڿ����ܶ�����Ӧѡ�������ſ������ռ����壬������������ˮ��β��������ˮ���գ�ע���ֹ�����ķ�����������ȷ������˳��Ϊ������װ�á�d��c��f��e��i��
�ʴ�Ϊ��d��c��f��e��i��
��a����ʯ���еμӰ�ˮ����ʯ���ܽ���ȣ�ʹ�����ų�������ȷ��
b����ʯ���е��백ˮ���ܽ���ȣ�ʹ�ų�����������ȷ��
C����������������ˮ�����ܷų���������ˮ��Ӱ�죬�������ɰ������ʴ���
D�����������Ͱ�ˮ��Ӧ�����������Σ��������ɰ������ʴ���
�ʴ�Ϊ��ab��
��NH3����������NO����Ⱦ������2�ֶԻ����������ʣ�Ӧ���ɵ�����ˮ���䷴Ӧ����ʽΪ6NO2+8NH3$\frac{\underline{\;����\;}}{\;}$7N2+12H2O���ɷ���ʽ��֪������7molN2��ת��24mol���ӣ���ÿ����0.5molN2ת��1.7mol���ӣ�
�ʴ�Ϊ��6NO2+8NH3$\frac{\underline{\;����\;}}{\;}$7N2+12H2O��1.7��
���� ���⿼�黷�������������ķ�����������ʵȡ��Լ�������ԭ��Ӧ����Ŀ�漰����֪ʶ����Ƕȹ㣬Ҫ��ѧ�����з����ͽ���������������Ŀ�Ѷ��еȣ�

 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д���������KMnO4��Һ��Ũ�ȿ�ѡ��0.010mol•L-1��0.0010mol•L-1��������������ѡ��0.5g��0g��ʵ���¶ȿ�ѡ��298K��323K��ÿ��ʵ��KMnO4������Һ��������Ϊ4mL��H2C2O4��Һ��0.10mol•L-1����������Ϊ2mL��
��1����֪������Һ�и�����������ʽ�ķֲ�����ͼ��ͼ1����д��KMnO4������Һ��H2C2O4��Һ��Ӧ�����ӷ���ʽ5H2C2O4+2MnO4-+6H+=1OCO2��+2Mn2++8H2O��

��2�����������ʵ����Ʊ�������ʵ��Ŀ��һ���������Ӧ��ʵ���ţ�
| ʵ���� | T/K | ����������/g | ����KMnO4��Һ��Ũ��/mol•L-1 | ʵ��Ŀ�� |
| �� | 298 | 0.5 | 0.010 | a��ʵ��ٺ͢�̽������KMnO4��Һ��Ũ�ȶԸ÷�Ӧ���ʵ�Ӱ�죻 b��ʵ��ٺ͢�̽���¶ȶԷ�Ӧ���ʵ�Ӱ�죻 c��ʵ��ٺ͢�̽�������Է�Ӧ���ʵ�Ӱ�죮 |
| �� | ||||
| �� | 0.010 | |||
| �� |
| ʵ���� | ��Һ��ɫ����ʱ�� t/min | ||
| ��1�� | ��2�� | ��3�� | |
| �� | 14.0 | 13.0 | 11.0 |
| �� | 6.5 | 6.7 | 6.8 |
�ڸ�ͬѧ�����������ݺ�ó���������������ͬ������£�����KMnO4��Һ��Ũ��ԽС������Ҫ��ʱ���Խ�̣��༴�䷴Ӧ����Խ�족�Ľ��ۣ�
����Ϊ�Ƿ���ȷ����ǡ���������Ϊ���þ������㣬ֱ�Ӹ��ݱ�����ɫʱ��ij��̾Ϳ����ж�Ũ�ȴ�С�뷴Ӧ���ʵĹ�ϵ������Ϊ�Ƿ���з���ǡ������������У�����Ϊ�������������ƿ���ֱ��ͨ���۲���ɫʱ��ij������жϵĸĽ�������ȡ�����������ͬ��Ũ�Ȳ�ͬ�IJ�����Һ���ֱ�ͬʱ�������ͬ��Ũ����ͬ�����Ը��������Һ��Ӧ��
��4���÷�Ӧ�Ĵ���ѡ��MnCl2����MnSO4������ѡ������ɣ�MnSO4����ѡ��MnCl2�������Ը�����ػ���Cl-��Ӧ������������Cl-��֤����Mn2+���˴�����
��5����ѧ�����кܶ࣬�������ͿƼ��������ش����ã�̽��С��������ͼ2װ��̽��MnO2��H2O2�ֽ�Ĵ�Ч������50mL H2O2һ���Լ���ʢ��0.10mol MnO2��ĩ����ƿ�У���ñ�״�����������ܶ�����������[V�������ܣ�/mL]��ʱ�䣨t/min���Ĺ�ϵ��ͼ3��ʾ��
��ʵ��ʱ�ų�������������110 mL��
��bС�ڣ�����ڡ���С�ڡ����ڡ���90mL��
| A�� | �Ȼ�ͭ��Һ�����۷�Ӧ��Cu2++Fe�TFe2++Cu | |
| B�� | ϡH2SO4�����۷�Ӧ��2Fe+6H+�T2Fe3++3H2�� | |
| C�� | ����������Һ��ϡH2SO4��Ӧ��Ba2++SO42-�TBaSO4�� | |
| D�� | ���Ȼ�����Һ��������ˮ��ӦFe3++3OH-�TFe��OH��3�� |
| A�� | Ũ��Ϊ0.1mol/L | B�� | Ũ��Ϊ1mol/L | C�� | ��NaOH 4g | D�� | ��NaOH 0.1mol |