��Ŀ����
����Ϊ�����䶳ѧ֮�������ء���͢��Robert Ettinger����1962��д���������ǰ������The Prospect Of Immortality��һ�顣���������о��˴�����ʵ��֤�����䶳����Ŀ��ܡ����磬��������͵͵����ﶬ�춼�����������������Զ�������н�������������Ϣ��ص���
| A���¶�Խ�ͣ���ѧ��ӦԽ�� | B�������·������˶� |
| C���¶Ƚ��ͣ���ѧ��Ӧֹͣ | D����ѧ��Ӧǰ�������غ� |
A
������������������¶ȵͣ���ѧ��Ӧ�������������¶������ߣ���Ӧ�����ӿ죬�ݴ˿�֪ѡ��A��ȷ����ѡA��
���㣺������������Է�Ӧ���ʵ�Ӱ��
�����������������ӱ�������ڵ���ѧ����ѧϰ��Ȥ������ѧ����ѧϰ��֪��������Ĺؼ�����ȷ�¶Ⱥͷ�Ӧ���ʵĹ�ϵ��Ȼ��������������ü��ɡ�

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д��ڷ�ӦC(s)��CO2(g)��2CO(g)�У���ʹ��Ӧ���ʼӿ����
������ѹǿ �������¶� �۽�C���� ��ͨCO2���� �ݼ�������ľ̿��
| A���٢ڢ� | B���٢ڢ� | C���٢ڢۢ� | D��ȫ�� |
���淴Ӧ��2NO2 2NO+O2���ܱ������з�Ӧ���ﵽƽ��״̬�ı�־�ǣ� ��
2NO+O2���ܱ������з�Ӧ���ﵽƽ��״̬�ı�־�ǣ� ��
�ٵ�λʱ��������n molO2��ͬʱ����2n molNO2
�ڵ�λʱ��������n molO2��ͬʱ������2n mol NO
����NO2��NO��O2�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ���ʵı�Ϊ2 : 2 : 1��״̬
�ܻ���������ɫ���ٸı��״̬
�ݻ��������ܶȲ��ٸı��״̬
�� ��������ƽ����Է����������ٸı��״̬
| A���٢ܢ� | B���ڢۢ� | C���٢ۢ� | D���٢ڢۢܢ� |
��10�֣�800�桢2L�ܱ�������Ӧ2NO(g)��O2(g) 2NO2(g)��ϵ�У� n(NO)��
2NO2(g)��ϵ�У� n(NO)��
ʱ��ı仯�����
| ʱ�䣨s�� | 0 | 1 | 2 | 3 | 4 | 5 |
| n(NO)(mol) | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
��1����Ӧ���е�2 sʱc (NO)= ��
��2����O2��ʾ��0��2s�ڸ÷�Ӧ��ƽ�����ʦԣ�___________��
 ��3�� ��Ӧ�ﵽƽ��״̬ʱNO��ת����= ������ʾ �� ��
��3�� ��Ӧ�ﵽƽ��״̬ʱNO��ת����= ������ʾ �� ����4���ж�һ���淴Ӧ�Ƿ�ﵽƽ��״̬�������кܶ࣬ijͬѧ��Ը÷�Ӧ���һ�����룺�ⶨ������������ܶȣ����ܶȲ��ٸı�ʱ�����жϳ��÷�Ӧ�Ѿ��ﵽƽ��״̬������Ϊ���������Ƿ���ȷ�� ����ǡ�����˵��������� ��
(14��)������һЩ���ʵ��۷е�����(��ѹ)��
| | �� | �� | Na2CO3 | ���ʯ | ʯī |
| �۵�(��) | 63.65 | 97.8 | 851 | 3550 | 3850 |
| �е�(��) | 774 | 882.9 | 1850(�ֽ����CO2) | --- | 4250 |
4Na(g)+3CO2(g)
 2Na2CO3(1)+C(s�����ʯ)����H��-1080��9kJ��mol
2Na2CO3(1)+C(s�����ʯ)����H��-1080��9kJ��mol(1)������Ӧ��ƽ�ⳣ������ʽΪ ����4v��(Na)��3v��(CO2)����Ӧ�Ƿ�ﵽƽ�� (ѡ��ǡ���)��
(2)����Ӧ��10L�ܱ���������ѹ�½��У��¶���890�����ߵ�1860�棬����Ӧʱ��Ϊ10min�������Ƶ����ʵ���������0��2mol����10min��CO2��ƽ����Ӧ����Ϊ ��
(3)��ѹ�������ڽ��ʯ���Ʊ������� ��
(4)��CO2(g)+4Na(g)��2Na2O(s)+C(s�����ʯ) ��H��-357��5kJ��mol����Na2O������C(���ʯ)��Ӧ�õ�Na(g)��Һ̬Na2CO3(1)���Ȼ�ѧ����ʽ�� ��
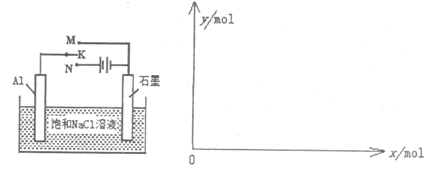
(5)��ͼ����K��Mʱ��ʯī�� �����缫��ӦʽΪ ����K��Nһ��ʱ������0.3mol����ת�ƣ�����y��x�仯��ͼ��x������n(H2O)������y������n[Al(OH)3]����Ӧ�������������й����ݡ�
 pC(g)+qD(g)��C���ʵ�����������w��C�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ�������ж���ȷ���ǣ� ��
pC(g)+qD(g)��C���ʵ�����������w��C�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ�������ж���ȷ���ǣ� ��
 2C��g���ﵽƽ��ʱ��A��B��C�����ʵ����ֱ�Ϊ4mol��2 mol��4 mol�������¶Ⱥ�ѹǿ���䣬��ƽ�����������ߵ����ʵ��������µ�������ʹƽ�����Ƶ��� �� ��
2C��g���ﵽƽ��ʱ��A��B��C�����ʵ����ֱ�Ϊ4mol��2 mol��4 mol�������¶Ⱥ�ѹǿ���䣬��ƽ�����������ߵ����ʵ��������µ�������ʹƽ�����Ƶ��� �� ��  NaNO3��s��+ClNO��g�� K1 ?H < 0 ��I��
NaNO3��s��+ClNO��g�� K1 ?H < 0 ��I�� Fe(s)��CO2(g)��ƽ�ⳣ��K��0.25��
Fe(s)��CO2(g)��ƽ�ⳣ��K��0.25�� HSO3��+OH��ˮ��ƽ�����ʵ�� ����ѡ����ĸ����
HSO3��+OH��ˮ��ƽ�����ʵ�� ����ѡ����ĸ���� C(g)��3D(s)�����ܱ��������ݻ����¶ȶ���ͬ�������£��ֱ����������;������ƽ�⣺��. A��B����ʼ���ʵ�����Ϊ2 mol����.C��D����ʼ���ʵ����ֱ�Ϊ2 mol��6 mol������˵������ȷ���� ����ѡ����ĸ����
C(g)��3D(s)�����ܱ��������ݻ����¶ȶ���ͬ�������£��ֱ����������;������ƽ�⣺��. A��B����ʼ���ʵ�����Ϊ2 mol����.C��D����ʼ���ʵ����ֱ�Ϊ2 mol��6 mol������˵������ȷ���� ����ѡ����ĸ����