МвДҝДЪИЭ
ЎҫМвДҝЎҝ»ҜәПОпI(C9H12O2)КЗәПіЙДі№ҰДЬёЯ·ЦЧУІДБПөДЦРјдМеЈ¬УЙC2H4әН![]() әПіЙөДВ·ПЯИзНјЛщКҫЈә
әПіЙөДВ·ПЯИзНјЛщКҫЈә

ТСЦӘЈәiЎўRCHO+CH3CHO![]() RCH=CHCHO+H2O(RОӘҙъұнМю»щ»тЗвФӯЧУ)
RCH=CHCHO+H2O(RОӘҙъұнМю»щ»тЗвФӯЧУ)
iiЎў![]()
»ШҙрПВБРОКМвЈә
(1)КФјБXөДГыіЖ______________________ЎЈ
(2)EөДҪб№№јтКҪОӘ______________________ЎЈ
(3)·ҙУҰўЬөДАаРНКЗ______________________ЎЈ
(4)DЦРЛщә¬өД№ЩДЬНЕГыіЖКЗ______________________ЎЈ
(5)Рҙіц·ҙУҰўЭөД»ҜС§·ҪіМКҪЈә_________________________________ЎЈ
(6)Н¬Кұ·ыәППВБРМхјюөДIөДН¬·ЦТм№№Ме№ІУР___________ЦЦ(І»ҝјВЗБўМеТм№№)Ј¬ЖдЦРәЛҙЕ№ІХсЗвЖЧНјұнГч·ЦЧУЦРУР5ЦЦЗвФӯЧУөДОӘ___________(Рҙ1ЦЦҪб№№јтКҪ)ЎЈa.·ЦЧУЦРә¬УРұҪ»·ЗТұҪ»·ЙПУРИэёцИЎҙъ»щЈ»b.1molУР»ъОпЧо¶аДЬПыәД2 mol NaOHЎЈ
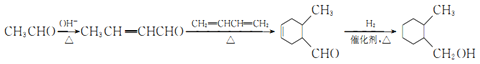
(7)ЙијЖТФТТИ©әН1Ј¬3-¶Ў¶юП©(CH2=CHCH=CH2)ОӘФӯБПЦЖұё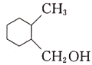 өДәПіЙВ·ПЯЈә________ (УГБчіМНјұнКҫЈ¬ОЮ»ъКФјБИОСЎ)ЎЈ
өДәПіЙВ·ПЯЈә________ (УГБчіМНјұнКҫЈ¬ОЮ»ъКФјБИОСЎ)ЎЈ
Ўҫҙр°ёЎҝјЧИ© CH2=CHCOOCH3 ПыИҘ·ҙУҰ МјМјЛ«јьЎўфИ»щ ![]() 12
12 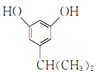 »т
»т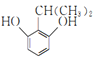
ЎҫҪвОцЎҝ
ёщҫЭәПіЙВ·ПЯНјЈәУЙA(CH2=CH2)Сх»ҜЙъіЙB(CH3CHO)Ј¬ёщҫЭТСЦӘМхјюўЩЈ¬CH3CHOУлHCHO·ҙУҰЙъіЙCЈЁCH2=CHCHOЈ©Ј¬CH2=CHCHOҙЯ»ҜСх»ҜЧӘ»ҜОӘD(CH2=CHCOOH)Ј¬CH2=CHCOOHУлјЧҙј·ўЙъхҘ»Ҝ·ҙУҰЙъіЙE(CH2=CHCOOCH3)Ј»FЈЁ![]() Ј©әНдеөДЛДВИ»ҜМјИЬТә·ўЙъјУіЙ·ҙУҰЙъіЙGЈЁ
Ј©әНдеөДЛДВИ»ҜМјИЬТә·ўЙъјУіЙ·ҙУҰЙъіЙGЈЁ![]() Ј©Ј¬G·ўЙъПыИҘ·ҙУҰўЬЙъіЙH(
Ј©Ј¬G·ўЙъПыИҘ·ҙУҰўЬЙъіЙH(![]() )Ј»УЙТСЦӘМхјюўЪҝЙЦӘЈ¬EУлH·ўЙъўЭІҪ·ҙУҰЙъіЙIЈ¬ЖдҪб№№јтКҪОӘ
)Ј»УЙТСЦӘМхјюўЪҝЙЦӘЈ¬EУлH·ўЙъўЭІҪ·ҙУҰЙъіЙIЈ¬ЖдҪб№№јтКҪОӘ![]() ЎЈ
ЎЈ
(1)ёщҫЭТФЙП·ЦОцЈ¬CH3CHOУлX·ҙУҰЙъіЙCЈЁCH2=CHCHOЈ©Ј¬ЛщТФКФјБXУҰОӘјЧИ©Ј»
№Кҙр°ёОӘЈәјЧИ©Ј»
(2)EөДҪб№№јтКҪОӘCH2=CHCOOCH3Ј»
№Кҙр°ёОӘЈәCH2=CHCOOCH3Ј»
(3)·ҙУҰўЬКЗВұҙъМюөДПыИҘ·ҙУҰЈ¬№К·ҙУҰўЬөД·ҙУҰАаРНКЗПыИҘ·ҙУҰЈ»
№Кҙр°ёОӘЈәПыИҘ·ҙУҰЈ»
(4) DОӘCH2=CHCOOHЈ¬Лщә¬өД№ЩДЬНЕГыіЖКЗМјМјЛ«јьЎўфИ»щЈ»
№Кҙр°ёОӘЈәМјМјЛ«јьЎўфИ»щЈ»
(5)·ҙУҰўЭОӘE(CH2=CHCOOCH3)әНH(![]() )·ўЙъ·ҙУҰЙъіЙI(
)·ўЙъ·ҙУҰЙъіЙI(![]() )Ј¬»ҜС§·ҪіМКҪОӘЈә
)Ј¬»ҜС§·ҪіМКҪОӘЈә![]() Ј»
Ј»
№Кҙр°ёОӘЈә![]() Ј»
Ј»
(6)IөДН¬·ЦТм№№МеЦР:a.·ЦЧУЦРә¬УРұҪ»·ЗТұҪ»·ЙПУРИэёцИЎҙъ»щЈ»b.1molУР»ъОпЧо¶аДЬПыәД2 mol NaOHЈ¬ёщҫЭIөД·ЦЧУКҪC9H12O2Ј¬№ІУР4ёцІ»ұҘәН¶ИЈ¬ЛөГчә¬УРұҪ»·УГИҘ4ёцІ»ұҘәН¶ИЈ¬»№ә¬УР2ёц·УфЗ»щәНТ»ёц-CH2CH2CH3»т-CH(CH3)2Ј¬№ІУР12ЦЦҪб№№ЈәўЩБҪёцфЗ»щҙҰУЪБЪО»өДУР2ЎБ2=4ЦЦЈ»ўЪБҪёцфЗ»щҙҰУЪјдО»өДУР2ЎБ3=6ЦЦЈ»БҪёцфЗ»щҙҰУЪ¶ФО»өДУР1ЎБ2=2ЦЦЈ»
ЖдЦРәЛҙЕ№ІХсЗвЖЧНјұнГч·ЦЧУЦРУР5ЦЦЗвФӯЧУөДУҰҫЯУРёЯ¶И¶ФіЖРФЈ¬ҝЙДЬОӘ »т
»т Ј»
Ј»
№Кҙр°ёОӘЈә »т
»т Ј»
Ј»
(7)УЙМвДҝЦРәПіЙВ·ПЯНјҝЙЦӘЈ¬ёщҫЭТСЦӘМхјюўЩЈ¬CH3CHOУлCH3CHOҝЙ·ҙУҰЙъіЙCH3CH=CHCHOЈ¬УЙТСЦӘМхјюўЪҝЙЦӘЈ¬1,3-¶Ў¶юП©ҝЙУлМјМјЛ«јь·ҙУҰЈ¬№КCH3CH=CHCHOУл1,3-¶Ў¶юП©·ҙУҰЙъіЙ Ј¬
Ј¬ јУЗв»№ФӯөГ
јУЗв»№ФӯөГ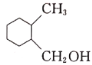 ЎЈ
ЎЈ
ҫЯМеәПіЙВ·ПЯОӘЈә Ј»
Ј»
№Кҙр°ёОӘЈә ЎЈ
ЎЈ

