��Ŀ����
����Ŀ���ڸ�����ع����ϵμ�Ũ���ᣬ���ϲ�������ɫ���塣��Ӧ�Ļ�ѧ����ʽΪ��2KMnO4 + 16HC1=2KC1 + 2MnCl2 + 5C12��+ 8H2O
�ش��������⣺
(1)�˷�Ӧ�е���������____�����ڱ�״���²���0.112L������ת�Ƶĵ�����Ϊ____mol��
(2)��ԭ�ӵĺ˵����Ϊ______����ԭ�ӵ�ԭ�ӽṹʾ��ͼ_______ ��
(3)��KC1�����д��ڵĻ�ѧ����__________��д��H2O����ʽ_________��
(4)��ҵ������������ʯ����ԭ������Ư�۾����˷�Ӧ�Ļ�ѧ����ʽ��_________��
(5)Ũ������������Ȼ��⣬��һ��ʵ��֤���Ȼ��⼫������ˮ��_________��
���𰸡�KMnO4 0.01 17 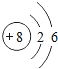 ���Ӽ�
���Ӽ� ![]() 2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O ��Ȫʵ��
2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O ��Ȫʵ��
��������
��1������������ԭ��Ӧ�Ĺ������𣻸���n=![]() ��������������ʵ�����Ȼ��������1mol����ת��2mol���Ӽ��㣻
��������������ʵ�����Ȼ��������1mol����ת��2mol���Ӽ��㣻
��2����ԭ�ӵĺ˵����=ԭ����������ԭ�ӵĺ˵����=����������������ԭ�ӵĺ�������Ų����ɻ�����ԭ�ӽṹʾ��ͼ��
��3���Ȼ���Ϊ���ӻ����ˮ����Ϊ���ۻ��������2��O-H����
��4��������ʯ���鷴Ӧ�����Ȼ��ơ�������ƺ�ˮ��
��5������HCl���ܽ��Խϴ���һ˼·���ʵ�顣
��1����Ӧ2KMnO4 + 16HC1=2KC1 + 2MnCl2 + 5C12��+ 8H2O�У�KMnO4��+7��MnԪ�صõ����ӱ���ԭ��Ϊ����������״���²���0.112L���������ʵ���Ϊ��![]() =0.005mol��ת�Ƶ��ӵ����ʵ���Ϊ��0.005mol��2=0.01mol��
=0.005mol��ת�Ƶ��ӵ����ʵ���Ϊ��0.005mol��2=0.01mol��
��2��ClΪ17��Ԫ�أ��˵����=ԭ������=17��Oԭ�ӵĺ˵����=�����������=8������ԭ�ӵĺ�������Ų����ɿ�֪����ԭ�ӽṹʾ��ͼΪ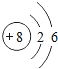 ��
��
��3��KClΪ���ӻ����ֻ�������Ӽ���H2OΪ���ۻ���������ʽΪ![]() ��
��
��4��������Ca(OH)2��Ӧ����CaCl2��Ca(ClO)2��ˮ����Ӧ�ķ���ʽΪ��2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O��
��5��ͨ����Ȫʵ���֤��HCl��������ˮ��