ЬтФПФкШн
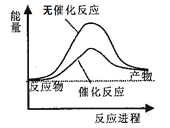
ЁОЬтФПЁПЃЈ1ЃЉВФСЯЪЧШЫРрРЕвдЩњДцЕФЮяжЪЛљДЁЃЌЪЧШЫРрЩчЛсНјВНЕФРяГЬБЎЁЃ
ЂйЯТСаЪєгкЙшЫсбЮВФСЯЕФЪЧ________(ЬюзжФИЃЌЯТЭЌ)ЁЃ
a. ЧрЛЈДЩЁЁЁЁЁЁЁЁЁЁЁЁb. ЧрЭЁЁЁЁЁЁЁЁЁЁЁЁЁЁc. СЄЧр
ЂкЮЊЗРжЙИжЬњЦїМўИЏЪДЃЌЯТСаОйДыВЛКЯРэЕФЪЧ________ЁЃ
a. БэУцЖЦаПЁЁ b. ЪЙгУВЛатИжЁЁ c. БэУцИНзХЭЦЌ
ЂлжЦдьВЃСЇКЭЫЎФрЕФжївЊдСЯжаЃЌОљгУЕНЕФдСЯЪЧ________ЁЃ
a. ЪЏЛвЪЏ b. ДПМю c. №ЄЭС
ЃЈ2ЃЉФГЦЗХЦЪГЦЗЕїСЯБъЧЉЕФвЛВПЗжШчгвЭМЁЃ

ЂйХфСЯжаЕФДѓСПАБЛљЫсЬЌЕЊРДдДгкЛЦЖЙжаЕФЕААзжЪЗЂЩњ________ЗДгІЕУЕНЁЃ
ЂкаЁТѓЗлжаЕФжївЊгЊбјЮяжЪЪЧ________ЁЃ
ЂлХфСЯжаЃЌЪєгкзХЩЋМСЕФЪЧ______ЃЛЪєгкЬ№ЮЖМСЕФЪЧ__________ЃЛЪєгкЗРИЏМСЕФЪЧ______ЁЃ
ЃЈ3ЃЉТЬЩЋЗЂеЙЁЂЕЭЬМЗЂеЙКЭбЛЗЗЂеЙЪЧЩњЬЌЮФУїНЈЩшЕФЛљБОЭООЖЁЃ
ЂйДгдДЫЎДІРэГЩздРДЫЎЃЌГЃашМгШыУїЗЏЁЂЛюадЬПДІРэвдМАЭЈТШЦјДІРэЕШЃЌЦфжаЭЈТШЦјДІРэЕФзїгУЪЧ__________________________ЁЃ
ЂкФПЧАЮвЙњПеЦјжЪСПМьВтЬхЯЕАќРЈЃКPM2.5ЁЂPM10ЁЂSO2ЁЂNO2ЁЂO3ЁЂCOСљЯюжИБъЁЃСљЯюжИБъжаЖдЮэіВЬьЦјЕФаЮГЩгаДйНјзїгУЁЂЮэіВЬьЦјгжФмНјвЛВНМгОчЦфЛ§ОлПХСЃЮяЕФЪЧ__________ЃЛв§Ц№ЫсгъЕФЦјЬхЮЊSO2КЭ________ЃЛУКжаМгШыЪЪСПЕФЪЏЛвЪЏПЩвдМѕЩйШМУКВњЩњЕФSO2ЃЌЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ______________________________________________ЁЃ
ЂлгУЖўбѕЛЏЬМЩњВњЛЏЙЄВњЦЗЃЌгаРћгкЖўбѕЛЏЬМЕФДѓСПЛиЪеЁЃCO2КЭH2дкДпЛЏМСМАИпЮТЁЂИпбЙЬѕМўЯТПЩвдКЯГЩввДМЃЌИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ___________________________________ЁЃ
ЁОД№АИЁП a c a ЫЎНт ЕэЗл НЙЬЧЩЋ Ш§ТШесЬЧ БНМзЫсФЦ ЯћЖОЩБОњ ЁОД№ЬтПе10ЁПPM2.5 NO2 2SO2ЃЋ2CaCO3ЃЋO2![]() 2CaSO4ЃЋ2CO2 2CO2ЃЋ6H2
2CaSO4ЃЋ2CO2 2CO2ЃЋ6H2![]() CH3CH2OHЃЋ3H2O
CH3CH2OHЃЋ3H2O
ЁОНтЮіЁП(1) ЂйЧрЛЈДЩЪЧ№ЄЭСьбЩеЖјГЩЕФЃЌЪєгкЙшЫсбЮВњЦЗЃЌЙЪaе§ШЗЃЛЧрЭЪЧКЯН№ЃЌЫљвдbДэЮѓЃЛСЄЧрЪєгкгаЛњЬўРрЛЏКЯЮяЃЌЙЪcДэЮѓЁЃе§ШЗД№АИЮЊaЃЛ ЂкaЁЂдкИжЬњЦїМўБэУцЖЦаПЃЌгЩгкаПБШЬњЛюЦУЃЌЫљвдаПБЛИЏЪДЖјБЃЛЄСЫИжЬњЃЌЙЪaКЯРэЃЛbЁЂгЩгкВЛатИжВЛвзБЛИЏЪДЃЌЙЪbКЯРэЃЛcЁЂдкИжЬњЦїМўБэУцИНзХЭЦЌЃЌгЩгкЬњБШЭЛюЦУЃЌЫљвдМгПьСЫИжЬњЕФИЏЪДЃЌЙЪcВЛКЯРэЁЃе§ШЗД№АИЮЊcЃЛЂлЙЄвЕЩЯЩњВњВЃСЇЕФдСЯЪЧЪЏЛвЪЏЁЂДПМюКЭЪЏгЂЃЌЩњВњЫЎФрЕФдСЯЪЧЪЏЛвЪЏКЭ№ЄЭСЃЌЫљвдЖўепЖМгУЕНЕФдСЯЪЧЪЏЛвЪЏЃЌМДе§ШЗД№АИЮЊaЃЛ
(2) ЂйЕААзжЪЫЎНтЕФзюжеВњЮяЪЧАБЛљЫсЃЌЫљвдХфСЯжаЕФДѓСПАБЛљЫсЬЌЕЊРДдДгкЛЦЖЙжаЕФЕААзжЪЗЂЩњЫЎНтЗДгІЕУЕНЃЛЂкаЁТѓЗлжаЕФжївЊгЊбјЮяжЪЕэЗлЃЌвђЕэЗлОЫЎНтзюжеВњЮяЮЊЦЯЬбЬЧЃЛЂлЗжЮіХфСЯжаЕФЮяжЪПЩжЊЃЌНЙЬЧЩЋЪЧгабеЩЋЕФЮяжЪЃЌЪєгкзХЩЋМСЃЛШ§ТШесЬЧОпгаЬ№ЮЖЃЌЫљвдЪєгкЬ№ЮЖМСЃЛБНМзЫсФЦОпгаЗРИЏадФмЃЌЫљвдЪєгкЗРИЏМСЃЛ
(3) Ђй ТШЦјШмгкЫЎЩњГЩЕФДЮТШЫсОпгаЧПбѕЛЏадЃЌПЩвдЦ№ЕНЩБОњЯћЖОзїгУЃЌЫљвдГЃгУгкздРДЫЎЕФЯћЖОЩБОњЃЛЂкетСљЯюжИБъжаЖдЮэіВЬьЦјЕФаЮГЩгаДйНјзїгУЁЂЮэіВЬьЦјгжФмНјвЛВНМгОчЦфЛ§ОлПХСЃЮяЕФЪЧPM2.5ЃЛв§Ц№ЫсгъЕФЦјЬхЮЊSO2КЭNO2ЃЛдкУКЕФШМЩеЪБЃЌЪЏЛвЪЏЗжНтЩњГЩCaOКЭCO2ЃЌCaOЪєгкМюадбѕЛЏЮяЃЌПЩгыЫсадбѕЛЏЮяSO2ЗДгІЩњГЩCaSO3ЃЌCaSO3гжШнвзБЛбѕЛЏЮЊCaSO4ЃЌДгЖјМѕЩйШМУКВњЩњЕФSO2ЃЌЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ2SO2ЃЋ2CaCO3ЃЋO2![]() 2CaSO4ЃЋ2CO2ЃЛЂлИљОнЬтФПа№ЪіПЩжЊЃЌЗДгІЮяЮЊCO2КЭH2ЃЌЩњГЩЮяЮЊввДМЃЌЫљвдЗДгІЕФЛЏбЇЗНГЬЪНЮЊ2CO2ЃЋ6H2
2CaSO4ЃЋ2CO2ЃЛЂлИљОнЬтФПа№ЪіПЩжЊЃЌЗДгІЮяЮЊCO2КЭH2ЃЌЩњГЩЮяЮЊввДМЃЌЫљвдЗДгІЕФЛЏбЇЗНГЬЪНЮЊ2CO2ЃЋ6H2 ![]() CH3CH2OHЃЋ3H2OЁЃ
CH3CH2OHЃЋ3H2OЁЃ

ЁОЬтФПЁПКЃДјКЌгаДѓСПЕФЕтЃЌУП1000gКЃДјжаКЌЕт5gзѓгвЁЃЪЕбщЪвжаЃЌДгКЃдхРяЬсШЁЕтЕФВПЗжСїГЬШчЯТЭМЁЃ

(1)ЂлЕФВйзїУћГЦЪЧ________________ЃЌЂнЕФВйзїУћГЦЪЧ__________________ЁЃ
(2)ЪдМСbПЩвдЪЧЫФТШЛЏЬМЃЌЛЙПЩвдЪЧ_________________________(ЬюУћГЦ)ЁЃ
(3)ЙигкЂнЕФВйзїВНжшЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ_____________________(ЬюзжФИ)ЁЃ
A. еёЕДвКЬхЪБЃЌашвЊЕЙзЊЗжвКТЉЖЗ
B. ГфЗжеёЕДвКЬхКѓЃЌНЋЗжвКТЉЖЗЗХжУдкЬњМмЬЈЩЯЃЌСЂМДЗжвК
C. ЗжвКЪБЃЌЩЯЯТВувКЬхЖМвЊДгЗжвКТЉЖЗЯТПкбизХЩеБФкБкСїШыВЛЭЌЕФЩеБ
D. ЗжвКЪБЃЌашвЊШћНјЗжвКТЉЖЗЩЯЗНЕФВЃСЇШћЃЌЪЙЗжвКТЉЖЗУмЗт
(4)ЪдМСaПЩбЁгУЯЁСђЫсЫсЛЏЕФЙ§бѕЛЏЧтШмвКЃЌВЙШЋВНжшЂмЗДгІЕФРызгЗНГЬЪНЃК___IЈD +____H2O2+______==____I2+______ЁЃ(ЯЕЪ§ЮЊЁА1ЁБЪБЃЌвЊаДЁА1ЁБ)
(5)ФГаЫШЄаЁзщЩшМЦЪЕбщЗНАИЃЌДгКЌI2ЕФCCl4ШмвКжаЗжРыI2КЭCCl4ЁЃвбжЊЃК
ШлЕу | ЗаЕу | |
I2 | 114Ёц | 184Ёц |
CCl4 | -23Ёц | 77Ёц |
ЂйаЁзщЭЌбЇИљОнзЪСЯЃЌВЩгУСЫеєСѓЕФЗНЗЈЃЌзщзАСЫШчЯТЭМзАжУЃЌвЧЦїBЕФУћГЦЪЧ_____ЃЌЭМжагавЛИіУїЯдДэЮѓЃЌгІИФе§ЮЊ_______________________________________ЁЃ
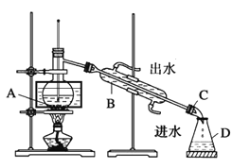
ЂкИУаЁзщЭЌбЇИФе§зАжУДэЮѓКѓЃЌНјааЪЕбщЁЃгУ80ЁцЫЎдЁМгШШЦЌПЬЃЌЙлВьЕНЩеЦПжаГіЯжзЯЩЋеєЦјЃЌзЖаЮЦПжавВПЊЪМЪеМЏЕНЧГзЯКьЩЋШмвКЃЌзюжеЩеЦПжаВаСєЩйСПЕФI2ЁЃЭЈЙ§ЪЕбщЕУГіНсТлЃЌГЃбЙЯТЕФеєСѓ__________________________(ЬюЁАЪЪКЯЁБЛђЁАВЛЪЪКЯЁБ)ЗжРыI2КЭCCl4ЁЃ
ЁОЬтФПЁПCO2ЕФРћгУЪЧЙњМЪЩчЛсЦеБщЙизЂЕФЮЪЬтЁЃ
ЃЈ1ЃЉCO2ЕФЕчзгЪНЪЧ______ЁЃ
ЃЈ2ЃЉCO2дкДпЛЏМСзїгУЯТПЩвджБНгзЊЛЏЮЊввЖўДМКЭМзДМЃЌЕЋШєЗДгІЮТЖШЙ§ИпЃЌввЖўДМЛсЩюЖШМгЧтЩњГЩввДМЁЃ
![]()
ЛёШЁввЖўДМЕФЗДгІРњГЬПЩЗжЮЊШчЯТ2ВНЃК
ЂёЃЎ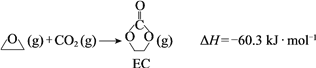
ЂђЃЎECМгЧтЩњГЩввЖўДМгыМзДМ
![]()
Ђй ВНжшЂђЕФШШЛЏбЇЗНГЬЪНЪЧ______ЁЃ
Ђк баОПЗДгІЮТЖШЖдECМгЧтЕФгАЯьЃЈЗДгІЪБМфОљЮЊ4аЁЪБЃЉЃЌЪЕбщЪ§ОнМћЯТБэЃК
ЗДгІЮТЖШ/ Ёц | ECзЊЛЏТЪ/ % | ВњТЪ/ % | |
ввЖўДМ | МзДМ | ||
160 | 23.8 | 23.2 | 12.9 |
180 | 62.1 | 60.9 | 31.5 |
200 | 99.9 | 94.7 | 62.3 |
220 | 99.9 | 92.4 | 46.1 |
гЩЩЯБэПЩжЊЃЌЮТЖШдНИпЃЌECЕФзЊЛЏТЪдНИпЃЌдвђЪЧ______ЁЃЮТЖШЩ§ИпЕН220 ЁцЪБЃЌввЖўДМЕФВњТЪЗДЖјНЕЕЭЃЌдвђЪЧ______ЁЃ
ЃЈ3ЃЉгУЯЁСђЫсзїЕчНтжЪШмвКЃЌЕчНтCO2ПЩжЦШЁМзДМЃЌзАжУШчЯТЭМЫљЪОЃЌЕчМЋaНгЕчдДЕФ______МЋЃЈЬюЁАе§ЁБЛђЁАИКЁБЃЉЃЌЩњГЩМзДМЕФЕчМЋЗДгІЪНЪЧ______ЁЃ

ЃЈ4ЃЉCO2НЯЮШЖЈЁЂФмСПЕЭЁЃЮЊЪЕЯжCO2ЕФЛЏбЇРћгУЃЌЯТСабаОПЗНЯђКЯРэЕФЪЧ______ЃЈЬюађКХЃЉЁЃ
aЃЎбЁдёИпФмСПЕФЗДгІЮяКЭCO2ЗДгІЛёЕУЕЭФмСПЕФЩњГЩЮя
bЃЎРћгУЕчФмЁЂЙтФмЛђШШФмЛюЛЏCO2Зжзг
cЃЎбЁдёИпаЇЕФДпЛЏМС