��Ŀ����
����A��B��C��D��E������ˮ�����ֻ��������ɵ��������±�����ÿ������ֻ��һ�Σ�
| ������ | Ag+��Na+��Fe3+��Al3+��Ba2+ |
| ������ | OH-��Cl-��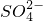 �� ��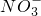 �� �� |
��C��Һ�Ի�ɫ��������Һ��Ϊ��ɫ��Һ��
����֪��B��C��D��E��Һ�ֱ����A��Һ��������ɫ������
��B��Һ������E��Һ��Ӧ���ɰ�ɫ�������������E��Һ����ɫ���������٣�������ȫ��ʧ��
��1���ݴ��ƶ����ǵĻ�ѧʽ��
A______��B______��C______��D______��E______��
��2��B��Һ��������E��Һ�����ӷ���ʽ______��
��3����C��Һ�μ�������D��Һ�����ӷ���ʽ______��
�⣺��1����C��Һ�Ի�ɫ��������Һ��Ϊ��ɫ��Һ������C�������������ӣ�ֻ�������ӿ��Ժ����������ӽ�ϣ�����������ֻ�ܺ��������ϣ�����A��������������������������ǿ���Ե���Һ�У�����E��ǿ�B�к��������ӣ�B��E��Ӧ����������������һ�ֳ���������B����������E�������������ϣ�ʣ���������ֻ�ܺ������ӽ�ϳ��Ȼ���������C���Ȼ�����Dֻ�����������ƣ�
�ʴ�Ϊ��AgNO3��Al2��SO4��3��FeCl3��Na2SO3��Ba��OH��2��
��2��������������������Һ��Ӧ��ʵ���ǣ�2Al3++3SO42-+3Ba2++6OH-=3BaSO4��+2Al��OH��3�����ʴ�Ϊ��2Al3++3SO42-+3Ba2++6OH-=3BaSO4��+2Al��OH��3����
��3�����������Ӿ��������ԣ�����������Ӿ��л�ԭ�ԣ��Ȼ�����Һ������������Һ��Ӧ��ʵ���ǣ�2Fe3++SO32-+H2O=
2Fe2++SO42-+2H+���ʴ�Ϊ��2Fe3++SO32-+H2O=2Fe2++SO42-+2H+��
�������Ի�ɫ����Һ�к��������ӣ����������������У�ֻ����������ӿ��Ժ������ӽ�ϣ������ӿ��Ժ��������������ӡ���������������֮�䷴Ӧ���ɳ���������������������ǿ���Ե���Һ�У�
������������һ�����ʵļ����⣬ע������֮��ķ�Ӧ�ǽ���Ĺؼ����ۺ��Խ�ǿ���Ѷȴ�
�ʴ�Ϊ��AgNO3��Al2��SO4��3��FeCl3��Na2SO3��Ba��OH��2��
��2��������������������Һ��Ӧ��ʵ���ǣ�2Al3++3SO42-+3Ba2++6OH-=3BaSO4��+2Al��OH��3�����ʴ�Ϊ��2Al3++3SO42-+3Ba2++6OH-=3BaSO4��+2Al��OH��3����
��3�����������Ӿ��������ԣ�����������Ӿ��л�ԭ�ԣ��Ȼ�����Һ������������Һ��Ӧ��ʵ���ǣ�2Fe3++SO32-+H2O=
2Fe2++SO42-+2H+���ʴ�Ϊ��2Fe3++SO32-+H2O=2Fe2++SO42-+2H+��
�������Ի�ɫ����Һ�к��������ӣ����������������У�ֻ����������ӿ��Ժ������ӽ�ϣ������ӿ��Ժ��������������ӡ���������������֮�䷴Ӧ���ɳ���������������������ǿ���Ե���Һ�У�
������������һ�����ʵļ����⣬ע������֮��ķ�Ӧ�ǽ���Ĺؼ����ۺ��Խ�ǿ���Ѷȴ�

��ϰ��ϵ�д�
�����Ŀ
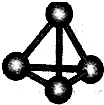 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��






