��Ŀ����
9������A��Ԫ�صĻ��������о�����������������Ҫ��;����ش��������⣺��1��Oԭ�ӻ�̬������ӵ��Ų�ʽΪ1s22s22p4��
��2����Na2O��SiO2��P2O5���������ﰴ�۷е��ɸߵ���˳������SiO2��Na2O��P2O5��
��3��SO32-��������ԭ�ӵ��ӻ���ʽsp3�������ӵ����幹��Ϊ�����ͣ�
��4��H2Se�ķе㣺-41.1�棬H2S�ķе㣺-60.4�棬�������߷е�������Ҫԭ����H2Se���Ӽ�������ǿ��H2S��
���� ��1������Oԭ�Ӻ�����8��������д��̬������ӵ��Ų�ʽ��
��2�����ݾ��������жϣ�һ��ԭ�Ӿ��壾���Ӿ��壾���Ӿ��壻
��3�����ݼۼ����������жϣ�
��4�����ݷ��Ӿ�����Ӽ�������ȡ������Է��������жϣ�
��� �⣺��1��Oԭ�Ӻ�����8�����ӣ���̬������ӵ��Ų�ʽΪ1s22s22p4���ʴ�Ϊ��1s22s22p4��
��2��SiO2Ϊԭ�Ӿ��壬�۷е�ȡ���ڹ��ۼ����ܣ��۷е�ܸߣ�Na2OΪ���Ӿ��壬�۷е�ȡ�����侧���ܣ��۷е�ϸߣ�P2O5Ϊ���Ӿ��壬�۷е�ȡ���ڷ��Ӽ����������۷е�ϵͣ��ʴ�Ϊ��SiO2��Na2O��P2O5��
��3��SO32-���Ӽ۵��Ӷ���Ϊ$\frac{6+2}{2}$=4���γ�4���µ��ӻ������������sp3�ӻ����µ��Ӷ���=�۵��Ӷ���-��λԭ����=4-3=1������һ�Թ¶Ե��ӣ�ռ��һ���ӻ��������S-O�����ų����ã�S��O���������ͣ��ʴ�Ϊ��sp3�������ͣ�
��4�����߾��ɷ��ӹ��ɣ��۷е�ȡ���ڷ��Ӽ������������Ӽ�������ȡ������Է���������H2Se����Է���������H2S�����H2Se���Ӽ�������ǿ��H2S��
�ʴ�Ϊ��H2Se���Ӽ�������ǿ��H2S��
���� �����ۺϿ������ʽṹ�����ʣ��漰��������Ų����ɡ��ӻ����ۡ����ӽṹ�ȣ������жϿռ乹�����׳�����

| A�� | pH=7��NaHSO3��Na2SO3�����Һ�У�c��Na+��=c��HSO3-��+c��SO32-�� | |
| B�� | �����ʵ���Ũ�ȵ�������Һ�У���NH4Al��SO4��2 ��NH4Cl ��CH3COONH4��NH3•H2O��c��NH4+���ɴ�С��˳���Ǣ٣��ڣ��ۣ��� | |
| C�� | 0.1 mol•L-1�Ĵ����pH=a��0.01 mol•L-1�Ĵ����pH=b����a+1=b | |
| D�� | 0.1 mol•L-1�Ĵ�������Һ20 mL��0.1 mol•L-1������10 mL��Ϻ���Һ�����ԣ����У�c��CH3COOH����c��Cl-����c��CH3COO-����c��H+����c��OH-�� |
| A�� | 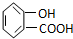 | B�� | CH3CH2=CHCOOH | C�� | 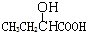 | D�� | 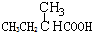 |
| A�� | Na2S��ˮ�⣺S2-+2H2O?H2S+2OH- | |
| B�� | NaHCO3�ĵ��룺NaHCO3 ?Na++H++CO32- | |
| C�� | HSO3-�ĵ��룺HSO3-?H++SO32- | |
| D�� | �����ĵ绯ѧ��ʴ�ĸ�����Ӧʽ��Fe-3e-�TFe3+ |
| A�� | ��֪��298Kʱ������Ӧ���й����ݣ�C��s��+$\frac{1}{2}$ O2��g���TCO��g����H1=-110.5 kJ•mol-1C��s��+O2��g���TCO2��g����H2=-393.5 kJ•mol-1����C��s��+CO2��g���T2CO��g����H=-172.5 kJ•mol-1 | |
| B�� | KI��Һ�еμӹ�����ˮ������Ӧ�����ӷ���ʽΪ3Cl2+I-+3H2O�T6H++IO3-+6Cl- | |
| C�� | �����ʵ�����NH4HCO3��Ba��OH��2 ��Һ��Ϸ�Ӧ�����ӷ���ʽΪBa2++HCO3-+OH-�TBaCO3��+H2O | |
| D�� | ��ͭ���缫���������Һ��2H2O$\frac{\underline{\;���\;}}{\;}$ 2H2��+O2�� |
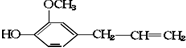 ����ܾ��е������ǣ����ܷ����ӳɷ�Ӧ������ʹ���Ը��������Һ��ɫ�����ܷ���ȡ����Ӧ�����������Ȼ���������ɫ��Ӧ�����ܷ����кͷ�Ӧ�����ܷ�����ȥ��Ӧ��������
����ܾ��е������ǣ����ܷ����ӳɷ�Ӧ������ʹ���Ը��������Һ��ɫ�����ܷ���ȡ����Ӧ�����������Ȼ���������ɫ��Ӧ�����ܷ����кͷ�Ӧ�����ܷ�����ȥ��Ӧ��������| A�� | ȫ�� | B�� | ���٢ڢۢ� | C�� | �����ⶼ�� | D�� | ���ܢ��ⶼ�� |

| A�� | �÷�ӦΪ���ȷ�Ӧ | |
| B�� | �÷�Ӧ��ΪBa��OH��2•8H2O��NH4Cl����ķ�Ӧ | |
| C�� | �÷�Ӧ��Ϊ�����������е�ȼ�շ�Ӧ | |
| D�� | �÷�Ӧֻ���ڼ��������²��ܽ��� |