��Ŀ����
����A��B��C��D��E��Fԭ�������������������Ԫ�أ�����λ��Ԫ�����ڱ���ǰ�����ڣ�BԪ��ԭ�ӵļ۲�����������ڲ����������2����DԪ��ԭ�ӵ�L���Ӳ���ֻ�����ԳɶԵ��ӣ�EԪ����FԪ�ش���ͬһ�������ڵ��壬���ǵ�ԭ���������3,��EԪ�صĻ�̬ԭ����4��δ�ɶԵ��ӡ���ش��������⣺
(1) EԪ�ػ�̬ԭ�ӵĺ���۲�����Ų�ʽΪ_________��
(2)��Ԫ�ط��ű�ʾB��C��D����Ԫ�صĵ�һ�������ɵ͵��ߵ�����_________��
(3) AԪ����B��CԪ�ؿ��γɻ�����B2A4��C2A4��
��B2A4�ĽṹʽΪ_________��
�������й�C2A4��˵����ȷ����_________��
a.һ���÷����к���4���Ҽ�
b.�÷��ӿ���Ϊ��λ���γ���λ��
c.�÷����ǷǼ��Է��� d.1mol�÷��������γ�4mol���
e.�÷��ӵ��ȶ����������
f.�÷�����C��ԭ�ӹ����sp3�ӻ�
(4)B���ʵ�һ�ֵľ���ṹ��ͼ����ʾ��E���ʵ�һ�ֵľ���ṹ��ͼ����ʾ��

��ͼ�еĵ���B������_________��ͼ����Bԭ�ӵ���λ����ͼ����Eԭ�ӵ���λ��֮_________��
EԪ����DԪ���γɵ�ED������NaCl����һ�������Ƚ�ED��NaCl�ľ����ܴ�С���迼�ǵ�������_______________________________________________________________________________��
(5)������������ʾ,F���ʵľ��������ж���,���侧���ֱ����������ܶѻ������������ѻ�����ʱ���䵥�ʵ��ܶ�֮��Ϊ_________��
(1) EԪ�ػ�̬ԭ�ӵĺ���۲�����Ų�ʽΪ_________��
(2)��Ԫ�ط��ű�ʾB��C��D����Ԫ�صĵ�һ�������ɵ͵��ߵ�����_________��
(3) AԪ����B��CԪ�ؿ��γɻ�����B2A4��C2A4��
��B2A4�ĽṹʽΪ_________��
�������й�C2A4��˵����ȷ����_________��
a.һ���÷����к���4���Ҽ�
b.�÷��ӿ���Ϊ��λ���γ���λ��
c.�÷����ǷǼ��Է��� d.1mol�÷��������γ�4mol���
e.�÷��ӵ��ȶ����������
f.�÷�����C��ԭ�ӹ����sp3�ӻ�
(4)B���ʵ�һ�ֵľ���ṹ��ͼ����ʾ��E���ʵ�һ�ֵľ���ṹ��ͼ����ʾ��

��ͼ�еĵ���B������_________��ͼ����Bԭ�ӵ���λ����ͼ����Eԭ�ӵ���λ��֮_________��
EԪ����DԪ���γɵ�ED������NaCl����һ�������Ƚ�ED��NaCl�ľ����ܴ�С���迼�ǵ�������_______________________________________________________________________________��
(5)������������ʾ,F���ʵľ��������ж���,���侧���ֱ����������ܶѻ������������ѻ�����ʱ���䵥�ʵ��ܶ�֮��Ϊ_________��
��ÿ��2�֣���(4)��1��1�֣�����15�֣���1��3d64s2��2��B��D��C
��3��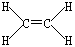 �� b��e��f��ȫ��2�֣�ѡ��1��2����1�֣�ѡ��һ�������÷֣�
�� b��e��f��ȫ��2�֣�ѡ��1��2����1�֣�ѡ��һ�������÷֣�
��4�����ʯ��1:2 �����ӵİ뾶�����������ĵ��
��5��3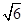 :8
:8
��3��
 �� b��e��f��ȫ��2�֣�ѡ��1��2����1�֣�ѡ��һ�������÷֣�
�� b��e��f��ȫ��2�֣�ѡ��1��2����1�֣�ѡ��һ�������÷֣���4�����ʯ��1:2 �����ӵİ뾶�����������ĵ��
��5��3
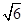 :8
:8���������BԪ��ԭ�ӵļ۲�����������ڲ����������2��������B��̼Ԫ�ء�DԪ��ԭ�ӵ�L���Ӳ���ֻ�����ԳɶԵ��ӣ����D����Ԫ�ء�C��ԭ����������B��D�м䣬��C�ǵ�Ԫ�ء�EԪ�صĻ�̬ԭ����4��δ�ɶԵ��ӣ���˵��EӦ���ǵ�������Ԫ�أ�������Cr��Fe��EԪ����FԪ�ش���ͬһ�������ڵ��壬���ǵ�ԭ���������3,����Eֻ����Fe��F��Cu��
��1������ԭ��������26�����ݹ���ԭ����֪����۵����Ų�ʽΪ3d64s2��
��2��BΪ̼Ԫ�أ�CΪ��Ԫ�أ�DΪ��Ԫ�أ�ͬ���ڵ�һ������������Ҿ����������ƣ����Ե�һ������O��C�����ڵ�Ԫ��ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�������ϵͣ���һ�����ܴ�������Ԫ�أ�����B��C��D����Ԫ�صĵ�һ��������ֵ��С�����˳��ΪC��O��N��
��3��AԪ����B��CԪ�ؿ��γɻ�����B2A4��C2A4����˵��AӦ������Ԫ�ء�
��B2A4����ϩ����ĽṹʽΪ
 ��
����C2A4�Ľṹʽ��
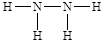 ������a. һ���÷����к���5���Ҽ���a����ȷ��b.�÷����е�Ԫ�غ��й¶Ե��ӣ����Կ���Ϊ��λ���γ���λ����b���ڣ�c.�÷��Ӳ��Գƣ��Ǽ��Է��ӣ�c����ȷ��d.���ڵ�Ԫ�ؿ����γ����������ƽ��1��N2H4���ӿ����γ�6��2��3����������1mol�÷��������γ�3mol�����d����ȷ��e.������ǻ�ѧ������Ӱ�����ʵ��ȶ��ԣ����÷��ӵ��ȶ���������أ�e��ȷ��f.�÷�����Nԭ�ӵ�ԭ�ӹ����sp3�ӻ���f��ȷ����ѡbef��
������a. һ���÷����к���5���Ҽ���a����ȷ��b.�÷����е�Ԫ�غ��й¶Ե��ӣ����Կ���Ϊ��λ���γ���λ����b���ڣ�c.�÷��Ӳ��Գƣ��Ǽ��Է��ӣ�c����ȷ��d.���ڵ�Ԫ�ؿ����γ����������ƽ��1��N2H4���ӿ����γ�6��2��3����������1mol�÷��������γ�3mol�����d����ȷ��e.������ǻ�ѧ������Ӱ�����ʵ��ȶ��ԣ����÷��ӵ��ȶ���������أ�e��ȷ��f.�÷�����Nԭ�ӵ�ԭ�ӹ����sp3�ӻ���f��ȷ����ѡbef����4�����ݾ����ļ�����֪�����ǽ��ʯ�����ݾ����ṹ��֪�����ʯ����λ����4�����������������ѻ�����λ����8�����Լ���Bԭ�ӵ���λ����ͼ����Eԭ�ӵ���λ��֮1:2��EԪ����DԪ���γɵ�ED������NaCl����һ�����������Ӿ��壬�������Ƚ�ED��NaCl�ľ����ܴ�С��Ӧ�ñȽϾ����ܴ�С������Ҫ���ǵ����������ӵİ뾶�����������ĵ�ɡ�
��5����������������ͭԭ�Ӹ�����1+8��
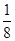 ��2����������������ʵ�ʺ��е�ͭԭ�Ӹ���=6��
��2����������������ʵ�ʺ��е�ͭԭ�Ӹ���=6��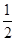 +8��
+8��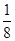 ��4����ͭԭ�Ӱ뾶��a���������ܶѻ������������ѻ��ı߳��ֱ�Ϊx��y����x2��2x2��(4a)2��y2��y2��(4a)2�����x��
��4����ͭԭ�Ӱ뾶��a���������ܶѻ������������ѻ��ı߳��ֱ�Ϊx��y����x2��2x2��(4a)2��y2��y2��(4a)2�����x�� ��y��
��y��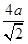 �����Ը���
�����Ը���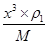 ��NA��2��
��NA��2��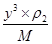 ��NA��4��֪�����ߵ��ܶ�֮�Ȧ�1:��2=3
��NA��4��֪�����ߵ��ܶ�֮�Ȧ�1:��2=3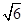 :8��
:8��
��ϰ��ϵ�д�
�����Ŀ


 + N2�� + 3CO2��
+ N2�� + 3CO2�� 


