��Ŀ����
����Ӧ����㷺�Ľ���֮һ�����Ļ�������������������Ҳ��������;��
��1������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ______�����������������ʢװNaOH��Һ��
��2��θ��ƽ������θ�ᣨ���ᣩ����ij���ҩ����к��е���Ч�ɷ�������������������ԭ���ǣ������ӷ���ʽ��ʾ����______��̼������Ҳ����������θ����࣬д����Ӧ�����ӷ���ʽ��______��
��3��Ϊ��ȫ������������Һ�е���Ԫ�أ������������______����Ӧ�����ӷ���ʽΪ______��
��4����5.4gAlͶ��200.0mL2.0mol?L-1��ij��Һ����������������ַ�Ӧ���н���ʣ�࣮����Һ����Ϊ______��
A��HNO3��ҺB��Ba��OH��2��ҺC��H2SO4��ҺD��HCl��Һ��
��1������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ______�����������������ʢװNaOH��Һ��
��2��θ��ƽ������θ�ᣨ���ᣩ����ij���ҩ����к��е���Ч�ɷ�������������������ԭ���ǣ������ӷ���ʽ��ʾ����______��̼������Ҳ����������θ����࣬д����Ӧ�����ӷ���ʽ��______��
��3��Ϊ��ȫ������������Һ�е���Ԫ�أ������������______����Ӧ�����ӷ���ʽΪ______��
��4����5.4gAlͶ��200.0mL2.0mol?L-1��ij��Һ����������������ַ�Ӧ���н���ʣ�࣮����Һ����Ϊ______��
A��HNO3��ҺB��Ba��OH��2��ҺC��H2SO4��ҺD��HCl��Һ��
��1�������������Ʒ�Ӧ����ƫ��������������������ʴ����Ӧ����ʽΪ��2Al+2NaOH+2H2O=2NaAlO2+3H2������������������ʢװNaOH��Һ��
�ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��2�������������������ԣ������ᷴӦ�����Ȼ�����ˮ����Ӧ���ӷ���ʽΪAl��OH��3+3H+=Al3++3H2O���ʿ�������θ����࣬
̼�����ƾ��������ԣ������ᷴӦ�����Ȼ��ơ�������̼��ˮ����Ӧ���ӷ���ʽΪ��HCO3-+H+=H2O+CO2�����ʿ�������θ����ࣻ
�ʴ�Ϊ��Al��OH��3+3H+=Al3++3H2O��HCO3-+H+=H2O+CO2����
��3�������������������������������ǿ�����ȫ������������Һ�е���Ԫ��ͨ���������ˮ���������백ˮ��Ӧ��������李�����������������Ӧ���ӷ���ʽΪ��Al3++3NH3?H2O=Al��OH��3��+3NH4+��
�ʴ�Ϊ����ˮ��Al3++3NH3?H2O=Al��OH��3��+3NH4+��
��4��5.4gAl�����ʵ���Ϊ0.2mol��
A��Al��HNO3��Һ��Ӧ����������������A�����ϣ�
B��200.0mL2.0mol?L-1��Ba��OH��2��Һ��n��OH-��=0.2L��2mol/L��2=0.8mol����
2Al+2OH-=+2H2O=2AlO2-+3H2����
0.8mol0.8mol
�����㣬����ʣ�࣬û����ʣ�࣬��B�����ϣ�
C��200.0mL2.0mol?L-1��H2SO4��Һ��n��H+��=0.2L��2mol/L��2=0.8mol����
2Al+6H+=2Al3++3H2��
0.2mol 0.6mol
�����㣬����ʣ�࣬û����ʣ�࣬��C�����ϣ�
D��200.0mL2.0mol?L-1��HCl��Һ��n��H+��=0.2L��2mol/L=0.4mol����
2Al+6H+=2Al3++3H2��
0.2mol 0.6mol
��㣬����ʣ�࣬��D���ϣ�
�ʴ�Ϊ��D��
�ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��2�������������������ԣ������ᷴӦ�����Ȼ�����ˮ����Ӧ���ӷ���ʽΪAl��OH��3+3H+=Al3++3H2O���ʿ�������θ����࣬
̼�����ƾ��������ԣ������ᷴӦ�����Ȼ��ơ�������̼��ˮ����Ӧ���ӷ���ʽΪ��HCO3-+H+=H2O+CO2�����ʿ�������θ����ࣻ
�ʴ�Ϊ��Al��OH��3+3H+=Al3++3H2O��HCO3-+H+=H2O+CO2����
��3�������������������������������ǿ�����ȫ������������Һ�е���Ԫ��ͨ���������ˮ���������백ˮ��Ӧ��������李�����������������Ӧ���ӷ���ʽΪ��Al3++3NH3?H2O=Al��OH��3��+3NH4+��
�ʴ�Ϊ����ˮ��Al3++3NH3?H2O=Al��OH��3��+3NH4+��
��4��5.4gAl�����ʵ���Ϊ0.2mol��
A��Al��HNO3��Һ��Ӧ����������������A�����ϣ�
B��200.0mL2.0mol?L-1��Ba��OH��2��Һ��n��OH-��=0.2L��2mol/L��2=0.8mol����
2Al+2OH-=+2H2O=2AlO2-+3H2����
0.8mol0.8mol
�����㣬����ʣ�࣬û����ʣ�࣬��B�����ϣ�
C��200.0mL2.0mol?L-1��H2SO4��Һ��n��H+��=0.2L��2mol/L��2=0.8mol����
2Al+6H+=2Al3++3H2��
0.2mol 0.6mol
�����㣬����ʣ�࣬û����ʣ�࣬��C�����ϣ�
D��200.0mL2.0mol?L-1��HCl��Һ��n��H+��=0.2L��2mol/L=0.4mol����
2Al+6H+=2Al3++3H2��
0.2mol 0.6mol
��㣬����ʣ�࣬��D���ϣ�
�ʴ�Ϊ��D��

��ϰ��ϵ�д�
�����Ŀ
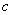 ��Mg2����Ϊ0.2mol?L��1,
��Mg2����Ϊ0.2mol?L��1,