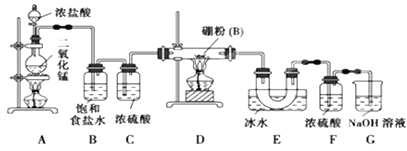
��Ŀ����
����Ŀ�����������ᡢ�����ᡢ˫��ˮ����ˮ��Ϊԭ�Ͽ��Ʊ���������泥�(NH4)3Fe(C6H5O7)2����
(1)Fe��̬��������Ų�ʽΪ___________��![]() ����Fe2+��λ��ԭ����________(��Ԫ�ط���)��
����Fe2+��λ��ԭ����________(��Ԫ�ط���)��
(2)NH3�����е�ԭ�ӵĹ���ӻ�������____________��C��N��OԪ�صĵ�һ�������ɴ�С��˳��Ϊ_______________��
(3)��NH![]() ��Ϊ�ȵ������һ�ַ���Ϊ_______________(�ѧʽ)��
��Ϊ�ȵ������һ�ַ���Ϊ_______________(�ѧʽ)��
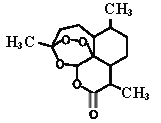
(4)������Ľṹ��ʽ��ͼ��1 mol�����������̼ԭ������ԭ���γɵ���������ĿΪ_________mol��
���𰸡�1s22s22p63s23p63d64s2��[Ar]3d64s2 O sp3 N��O��C CH4��SiH4 7
��������
(1)Fe������26�����ӣ�H2O��Oԭ���й¶Ե��ӣ��ṩ�¶Ե��ӡ�
(2)�ȼ���NH3�����е�ԭ�Ӽ۲���Ӷ�����ͬ���ڣ������ң���һ�����ܳ���������ƣ�����IIA����ڵ�IIIA�壬��VA����ڵ�VIA�塣
(3)���ݼ۵�����Si��C��N+�Ĺ�ϵ�ó�![]() ��Ϊ�ȵ�����ķ��ӡ�
��Ϊ�ȵ�����ķ��ӡ�
(4)�Ȼ��Ľṹ��![]() ��һ���Ȼ�����̼ԭ������ԭ�ӷֱ��γ�����������һ���ǻ���̼ԭ�������γ�һ��������
��һ���Ȼ�����̼ԭ������ԭ�ӷֱ��γ�����������һ���ǻ���̼ԭ�������γ�һ��������
(1)Fe������26�����ӣ����̬��������Ų�ʽΪ1s22s22p63s23p63d64s2��[Ar]3d64s2������H2O��Oԭ���й¶Ե��ӣ����[Fe(H2O)6]2+����Fe2+��λ��ԭ����O���ʴ�Ϊ��1s22s22p63s23p63d64s2��[Ar]3d64s2��O��
(2)NH3�����е�ԭ�Ӽ۲���Ӷ���Ϊ![]() ����˵��ӻ�����Ϊsp3��ͬ���ڣ������ң���һ�����ܳ���������ƣ�����IIA����ڵ�IIIA�壬��VA����ڵ�VIA�壬���C��N��OԪ�صĵ�һ�������ɴ�С��˳��ΪN��O��C���ʴ�Ϊ��sp3��N��O��C��
����˵��ӻ�����Ϊsp3��ͬ���ڣ������ң���һ�����ܳ���������ƣ�����IIA����ڵ�IIIA�壬��VA����ڵ�VIA�壬���C��N��OԪ�صĵ�һ�������ɴ�С��˳��ΪN��O��C���ʴ�Ϊ��sp3��N��O��C��
(3)���ݼ۵�����Si��C��N+���ó�![]() ��Ϊ�ȵ�����ķ�����CH4��SiH4���ʴ�Ϊ��CH4��SiH4��
��Ϊ�ȵ�����ķ�����CH4��SiH4���ʴ�Ϊ��CH4��SiH4��
(4)�Ȼ��Ľṹ��![]() ��һ���Ȼ�����̼ԭ������ԭ�ӷֱ��γ����������������Ȼ���6��������һ���ǻ���̼ԭ�������γ�һ�����������1mol�����������̼ԭ������ԭ���γɵ���������ĿΪ7mol���ʴ�Ϊ��7��
��һ���Ȼ�����̼ԭ������ԭ�ӷֱ��γ����������������Ȼ���6��������һ���ǻ���̼ԭ�������γ�һ�����������1mol�����������̼ԭ������ԭ���γɵ���������ĿΪ7mol���ʴ�Ϊ��7��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������﮵���������������(LiMn2O4)����ȡ���㷺ʹ�õ�LiCoO2����ҵ����ij���̿�(��Ҫ�ɷ�ΪMnO2�����������������������������)Ϊԭ���Ʊ�����﮵�������ͼ��
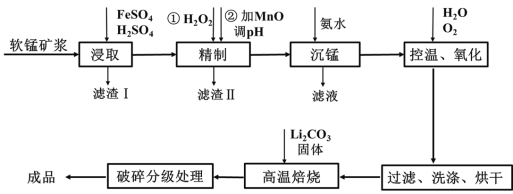
��֪�� lg2=0.3��![]() =8��25���й����ʵ��ܶȻ�������
=8��25���й����ʵ��ܶȻ�������
���� | Fe(OH)2 | Fe(OH)3 | Al(OH)3 | Mn(OH)2 |
Ksp | 8.0��10-16 | 4.0��l0-38 | 5.12��10-33 | 4.0��l0-14 |
(1)��֪﮵�طŵ�ʱ�����ĵ缫��ӦʽΪ��LiMn2O4+e-+Li+ ===Li2Mn2O4�������������Ԫ�صĻ��ϼ�Ϊ____��
(2)����ȡ���õ��Ľ�ȡҺ����������Ҫ��Mn2+������Mn2+�����ӷ���ʽΪ_____�����黹����Fe2+�ķ�����______��
(3)���������м���H2O2����������ֵ��Ķ࣬����Ҫԭ����______����������Һ�������ӵ�Ũ��Ϊ1 mol��L-1������������̵���pH�ķ�ΧΪ____(����Һ������Ũ��С��10-5 mol��L-1ʱ������Ϊ������ȫ)��
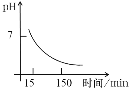
(4)���������õ�����Mn(OH)2��Mn2(OH)2SO4�˱������߾��ɱ�����ΪMn3O4������������ʱ��Һ��pH��ʱ��ı仯����ͼ����15~150 min���˱���һ���μӷ�Ӧ�ijɷ���______���жϵ�������____(�û�ѧ����ʽ��ʾ)��
(5)д������������������﮵Ļ�ѧ����ʽ_______��
����Ŀ��(1)��������������Ԫ�أ�Ŀǰ���۲���Ӫ��Ʒ�϶࣬ij�о���ѧϰС��Բ����������ĺ��������˲ⶨ���ṩҩƷ��FeCl2��Һ(dz��ɫ)��FeCl3��Һ(��ɫ)�����ۡ�ͭ�ۡ�KSCN��Һ����ˮ��������ѡ��
�ٽ��������е�Fe2��ת����Fe3�����������ѡ���Լ�д����ص����ӷ���ʽ��______��
������Fe3��ת����Fe2�����������ѡ���Լ�д����ص����ӷ���ʽ��__________��
(2)������ά����C����ʹʳ���е����������ӻ�ԭ�ɶ��������ӡ���仰ָ��ά����C����һ��Ӧ����____��������____�ԡ�
(3)ijͬѧ������Ԫ�ؼ�̬�ص㣬�������һ���۵㣺���������۷�Ӧ����Cl2����ʱ����FeCl3������������ʱ������FeCl2��Ϊ��֤�ù۵��Ƿ���ȷ����ͬѧ��һ������������Cl2ǡ����ȫ��Ӧ�õ�һ�������ʣ�Ȼ��ͨ��ʵ��ȷ����ɷ֡�̽���������£�
��������裺
����1���ù���������FeCl2��
����2���ù���������_______��
�����ʵ�鷽����
ȡ���������������ձ��У�������ˮ�ܽ⣬�õ���ҺA��Ȼ��ȡ����A��Һ�ֱ����ʵ�飬ʵ��������������±���
ʵ�鷽�� | ʵ������ | ���� |
��A��Һ�м�KSCN��Һ | _______ | ������������FeCl3 |
�����Ը��������Һ�м�����A��Һ | ���Ը��������Һ��ɫ�����Ա仯 | _______ |
�۸�������ʵ����ۣ�д��Cl2�����ۼ���ʱ������Ӧ�Ļ�ѧ����ʽ__________��