��Ŀ����
����Ŀ��һ�������ºϳ���ϩ�� 6 H2(g) +2CO2(g)![]() CH2=CH2(g) +4H2O(g)����֪�¶ȶ�CO2��ƽ��ת���ʺʹ�����Ч�ʵ�Ӱ����ͼ������˵����ȷ����
CH2=CH2(g) +4H2O(g)����֪�¶ȶ�CO2��ƽ��ת���ʺʹ�����Ч�ʵ�Ӱ����ͼ������˵����ȷ����
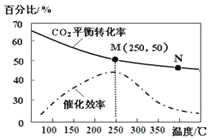
A. ������ϩ�����ʣ�v(M)һ��С��v(N)
B. ��ѧƽ�ⳣ����KN��K M
C. ���¶ȸ���250��ʱ�������¶ȣ�ƽ�����淴Ӧ�����ƶ����Ӷ�ʹ�����Ĵ�Ч�ʽ���
D. ��Ͷ�ϱ�n��H2����n��CO2��=3��1����ͼ��M��ʱ����ϩ���������Ϊ7.7��
���𰸡�D
������������A��M����¶ȵ���N����¶ȣ���M��Ĵ�Ч�ʸ���N��ģ�����������ϩ�����ʣ�v(M)��һ��С��v(N)��A����B�������¶ȣ�CO2��ƽ��ת���ʽ��ͣ�˵�����£�ƽ�������ƶ�������ӦΪ���ȷ�Ӧ����ѧƽ�ⳣ����KN<K M��B����C�������Ĵ�Ч����һ���¶�����ߣ�ƽ�����淴Ӧ�����ƶ���������Ĵ�Ч�ʽ����أ�C����D����Ͷ�ϱ�n��H2����n��CO2��=3��1����ͼ��M��ʱ��CO2��ƽ��ת������50%��
6 H2(g) +2CO2(g)![]() CH2=CH2(g) +4H2O(g)
CH2=CH2(g) +4H2O(g)
��ʼ����mol�� 3a a 0 0
�仯����mol�� 1.5a 0.5a 0.25a a
ƽ������mol�� 1.5a 0.5a 0.25a a
����ϩ���������Ϊ0.25a/��1.5a+0.5a+0.25a+a����100%=7.7����D��ȷ����ѡD��

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�����Ŀ����������������˫������������̼ԭ������ϵ���ԭ��������̼ͬԭ������Ӧ������������ԭ�����������һ���IJ����Ͷ�![]() ��
��![]() ��ʾ
��ʾ![]() ���±��г��������IJ����Ͷȣ�
���±��г��������IJ����Ͷȣ�
�л��� | ��ϩ | ��Ȳ | ������ | �� |
| 1 | 2 | 1 | 4 |
�ݴ�����˵������ȷ����![]() ����
����![]()
A.![]() �IJ����������ٽ��6molH���ﵽ����
�IJ����������ٽ��6molH���ﵽ����
B.![]() ��
��![]() ����6
����6
C.![]() �뻷����IJ����Ͷ���ͬ
�뻷����IJ����Ͷ���ͬ
D.![]() �IJ����Ͷ���
�IJ����Ͷ���![]() ��
��![]() �IJ����Ͷ���ͬ
�IJ����Ͷ���ͬ