��Ŀ����
6����ͼ��ʾ���� 500mL 0.100mol•L-1 Na2CO3��Һ�ļ����ؼ�ʵ�鲽��Ͳ�������ͼ�ش��������⣺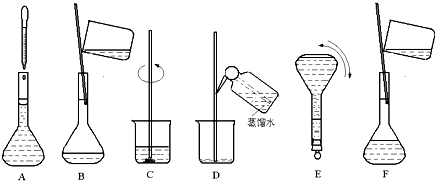
��1����������ҪNa2CO3����5.3g
��2������E�н�һ�����������µߵ����Σ�������������������ƿ��
��3������Bͨ����Ϊת�ƣ�����Aͨ����Ϊ���ݣ�
��4��������ʵ�鲽��A��F��ʵ������Ⱥ��������CBDFAE��
��5�����д��������ʹ��������ҺŨ��ƫ�ߵ���C������ĸ��
A������ƿϴ�Ӻ�δ���� B��ҡ�Ⱥ��Һ���½����ټ�ˮ���̶���
C������ʱ��������ƿƿ���̶��� D���ܽ����ʱ��Һ��ɽ���
���� ��1������m=nM=cvM���㣻
��2������һ�����ʵ���Ũ����Һ������������ƿ��
��3���ý�ͷ�ιܵμ���Һ�IJ��������Ƕ��ݣ�
��4������������Һ��ʵ��������̽���ʵ�鲽������
��5������c=$\frac{n}{V}$�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��� �⣺��1��ʵ��������500mL 0.100mol•L-1 Na2CO3��Һ��ҪNa2CO3������Ϊ��0.5L��0.1mol/L��106g/mol=2.9g���ʴ�Ϊ��5.3��
��2������һ�����ʵ���Ũ����Һ������������ƿ�����Ը�����������������ƿ���ʴ�Ϊ������ƿ��
��3����ˮ��Һ�����̶���1��2cmʱ���ý�ͷ�ι�������ƿ�еμ���Һ���ò����������Ƕ��ݣ��ʴ�Ϊ�����ݣ�
��4�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������ʵ������Ⱥ��������Ϊ��CBDFAE���ʴ�Ϊ��CBDFAE��
��5��A����������ƿ������ˮϴ�Ӻ�û�и�����ʵ����ʵ�������Һ�������δ�ı䣬������Ӱ�죻
B��ҡ�Ⱥ���Һ����ڿ̶��ߣ���������ˮ���̶��ߣ���Һ�����ƫ����������Һ��Ũ��ƫ�ͣ�
C������ʱ��������ƿƿ���̶��ߣ�������Һ�����ƫС��������Һ��Ũ��ƫ�ߣ�
D���ܽ����ʱ��Һ��ɽ����������ʵ����ʵ���ƫС������������Һ��Ũ��ƫ�ͣ�
��ѡC��
���� ���⿼��һ�����ʵ���Ũ����Һ�����ƣ��ѶȲ���ע��ʵ�鲽�衢������������ƿ��ѡȡ��Ϊ�״��㣮

 ��У����ϵ�д�
��У����ϵ�д�| NaCl | MgCl2 | CaCl2 | SiCl4 | |
| �۵㣨�棩 | 801 | 712 | 782 | -68 |
| �е㣨�棩 | 1 465 | 1 412 | 1 600 | 57.6 |
��CaCl2�������Ӿ��� ��SiCl4�Ƿ��Ӿ���
��1500��ʱ��NaCl�������� ��MgCl2ˮ��Һ���ܵ��磮
| A�� | ���� | B�� | ���� | C�� | �٢ڢ� | D�� | �٢ڢ� |
| A�� | Br2+2NaI�T2NaBr+I2 | B�� | Zn+H2SO4�TZnSO4+H2�� | ||
| C�� | 2C+SiO2$\frac{\underline{\;\;��\;\;}}{\;}$Si+2CO�� | D�� | 4Al+3MnO2 $\frac{\underline{\;\;��\;\;}}{\;}$3Mn+2Al2O3 |
| A�� | �յ���Ƕп�飬п���������Է����屻��ʴ | |
| B�� | �ڳ�ʪ�����Ի����У������ĵ绯ѧ��ʴ��Ҫ�����ⸯʴ | |
| C�� | ������������������� | |
| D�� | �ɽ��������ֹ������ֱ����Դ�����������Ա��������ܸ�ʴ |
| A�� | H2SO4 | B�� | Cl2 | C�� | CaCO3 | D�� | CO2 |
 ���ݻ���Ϊ500mL�Ģ������ܱ���������������㶨���䣩�зֱ����1molN2��2.5molH2�����������ķ�Ӧ�¶ȷֱ�ΪT1��T2��T3���Һ㶨���䣮������������ͬ������·�����Ӧ��N2+3H2?2NH3��H��0��ʵ���÷�Ӧ�����е�t minʱN2�����������ͼ��ʾ������˵����ȷ���ǣ�������
���ݻ���Ϊ500mL�Ģ������ܱ���������������㶨���䣩�зֱ����1molN2��2.5molH2�����������ķ�Ӧ�¶ȷֱ�ΪT1��T2��T3���Һ㶨���䣮������������ͬ������·�����Ӧ��N2+3H2?2NH3��H��0��ʵ���÷�Ӧ�����е�t minʱN2�����������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ��v��H2��=3v��N2��ʱ������˵�����������еķ�Ӧ��ƽ��״̬ | |
| B�� | ��t minʱ��һ���ﻯѧƽ��״̬����III | |
| C�� | ��t minʱ��â���c��N2��=1mol•L-1����������г���1.5molN2��1molNH3��H2��ת�������� | |
| D�� | ���������еķ�Ӧ���ﵽƽ����������������� |
| A�� | 100mL | B�� | 40mL | C�� | 50mL | D�� | 30mL |
 �������仯�����й㷺��Ӧ�ã�
�������仯�����й㷺��Ӧ�ã�