��Ŀ����
����Ŀ���������ڼ�չ��տɴ����ȷ��������������ø���(Al��Al2O3��Cr2O3��)�н�����������ʵ�ָ��������������á��乤���������£�
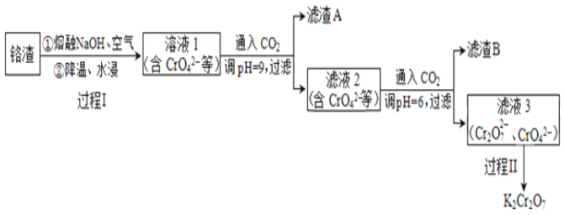
(1)���ȷ�ұ�����������������˽�������______(������ԡ���ԭ�ԡ�)��
(2)��Һ1�е���Ҫ����������CrO42-��_____(�����ӷ���)��
(3)����I����Cr2O3����ķ�Ӧ�У�������0.4 molCrO42-,���������������ʵ�����_______��
(4)ͨ��CO2������ҺpHʵ�����ʵķ��롣
������A���յõ�Al2O3�����õ�ⷨұ��Al��ұ��Al�Ļ�ѧ����ʽ��_______��
������B���ȷֽ��������ʿ���ѭ�����ã�B��________(�ѧʽ)��
����֪��2CrO42-+2H+![]() Cr2O72-+H2O K=4.0��1014
Cr2O72-+H2O K=4.0��1014
��Һ3��Cr2O72-��Ũ����0.04 mol/L����CrO42-��Ũ����_____mol/L��
(5)����II��Ŀ���ǵõ�K2Cr2O7��Ʒ����Ʒ���ؽᾧ���Ƶô�����K2Cr2O7��
��ͬ�¶��»�������ܽ��(g/100gH2O)
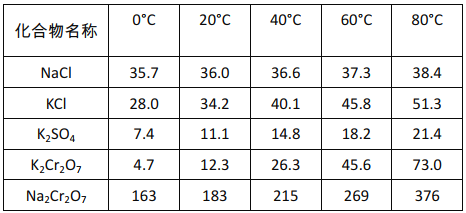
��ϱ������ݷ���������II�õ�K2Cr2O7��Ʒ�IJ����ǣ�______�����˵õ�K2Cr2O7��Ʒ��
���𰸡� ��ԭ�� AlO2-��OH- 0.3 mol 2Al2O3(����) ![]() 3O2 ��+ 4Al NaHCO3 0.01 ����Һ���м���Ũ�����KCl���������Ũ�������½ᾧ
3O2 ��+ 4Al NaHCO3 0.01 ����Һ���м���Ũ�����KCl���������Ũ�������½ᾧ
���������������ڼ�չ��տɴ����ȷ��������������ø�����Al��Al2O3��Cr2O3�ȣ��н�����������������������NaOH������������ˮ���������������ܽ�����ƫ�����ƣ�Cr2O3�ܽ�õ�CrO42-���õ���Һ���к���CrO42-��AlO2-��OH-��ͨ�������̼������ҺpH=9��������������������������������AΪ������������Һ2����ͨ�������̼������ҺpH=6���˵õ�������BΪ̼�����ƾ��壬��Һ3��Cr2O72-��CrO42-�ȣ����̢��Ŀ���ǵõ�K2Cr2O7��Ʒ����Ʒ���ؽᾧ���Ƶô�����K2Cr2O7���ݴ˽��
��1�����ȷ�ұ������������Ԫ�ػ��ϼ���0�۱仯Ϊ+3�ۣ�ʧȥ���ӱ��������������˽������Ļ�ԭ�ԣ�
��2��������������NaOH������������ˮ���������������ܽ�����ƫ�����ƣ�Cr2O3�ܽ�õ�CrO42-����˵õ�����Һ���к���CrO42-��AlO2-��OH-��
��3����Cr2O3����ķ�Ӧ�У���Ӧ�����ӷ���ʽΪ��2Cr2O3+8OH-+3O2=4CrO42-+4H2O��������0.4molCrO42-����Ӧ���������������ʵ���Ϊ0.3mol��
��4��������A���յõ�Al2O3�����õ�ⷨұ��Al��ұ��Al�Ļ�ѧ����ʽ��2Al2O3(����)![]() 3O2��+4Al��
3O2��+4Al��
������BΪ̼�����ƣ����ȷֽ���������̼���ƿ���ѭ�����ã����B��NaHCO3��
����֪2CrO42-+2H+![]() Cr2O72-+H2O��K=4.0��1014����Һ3��Cr2O72-��Ũ����0.04mol/L��K=c(Cr2O72-)/[c2(CrO42)��c2(H+)]��������Ũ��Ϊ10-6mol/L�����CrO42-��Ũ��Ϊ
Cr2O72-+H2O��K=4.0��1014����Һ3��Cr2O72-��Ũ����0.04mol/L��K=c(Cr2O72-)/[c2(CrO42)��c2(H+)]��������Ũ��Ϊ10-6mol/L�����CrO42-��Ũ��Ϊ![]() mol/L��0.01mol/L��
mol/L��0.01mol/L��
��5�����̢��Ŀ���ǵõ�K2Cr2O7��Ʒ����Ʒ���ؽᾧ���Ƶô�����K2Cr2O7���������ʵ��ܽ�����¶ȵĹ�ϵ��֪���̢�õ�K2Cr2O7��Ʒ�IJ����ǣ�����Һ���м���Ũ�����KCl���������Ũ�������½ᾧ��

 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�����Ŀ���ס��ҡ�������������ת����ϵ��ͼ��ʾ(��Ӧ������ȥ,��ͷ��ʾһ��ת��)�����и���������,��������ͼʾת����ϵ����

�� | �� | �� | �� | |
A | Cu | FeCl3��Һ | CuCl2��Һ | Fe |
B | H2O | Fe | H2 | O2 |
C | Al | NaOH��Һ | H2 | Al2O3 |
D | CH3CH2OH | O2 | CH3CHO | H2 |
A. A B. B C. C D. D