ĚâÄżÄÚČÝ
ˇľĚâÄżˇżÁňËáĘÔĽÁĆż±ęÇ©ÉϵÄÄÚČÝČçÍĽËůĘľ:

Ł¨1Ł©¸ĂÁňËáµÄÎďÖʵÄÁżĹ¨¶ČĘÇ____________mol/LŁ»
Ł¨2Ł©Äł»ŻŃ§ĐËȤС×é˝řĐĐÁňËáĐÔÖʵÄʵŃéĚ˝ľżĘ±,ĐčŇŞ240mL 4.6molŁ®L-1µÄϡÁňËá,ÔňĹäÖĆϡÁňËáʱĐčҪȡ____mLµÄ¸ĂÁňËá;ĹäÖĆʱĐčѡÓõÄŇÇĆ÷Ö÷ŇŞÓĐÁżÍ˛ˇ˘ÉŐ±ˇ˘˛ŁÁ§°ôˇ˘_______ˇ˘˝şÍ·µÎąÜŁ¨ÔÚşáĎßÉĎĚîĐ´ËůȱŇÇĆ÷µÄĂűłĆŁ©ˇŁ
Ł¨3Ł©ĹäÖĆąýłĚÖĐ,ĎÂÁвŮ×÷»áĘąĹäÖƵÄϡÁňËáČÜҺŨ¶ČĆ«¸ßµÄĘÇ____Ł¨ĚîĐňşĹŁ©ˇŁ
˘ŮÁżČˇĹ¨ÁňËáµÄÁżÍ˛ÓĂŐôÁóˮϴµÓ2~3´Î,˛˘°ŃĎ´µÓҺתČëČÝÁżĆż
˘ÚČÝÁżĆżĘąÓĂʱδ¸ÉÔď
˘ŰČÜ˝âşóδľŔäČ´ľÍŇĆŇş˛˘¶¨ČÝ
˘Ü¶¨ČÝʱ˛»ĐˇĐÄÓĐÉŮÁżŐôÁóË®µÎµ˝ĆżÍâ
˘Ý¶¨ČÝşóľŐńµ´ˇ˘ŇˇÔȡ˘ľ˛ÖĂ,·˘ĎÖŇşĂćµÍÓڿ̶ČĎߣ¬ÔŮĽÓŐôÁóË®˛ąÖÁżĚ¶ČĎß
ˇľ´đ°¸ˇż18Ł®462Ł®5250 mLČÝÁżĆż˘Ů˘Ű
ˇľ˝âÎöˇż
(1).¸ĂÁňËáµÄÎďÖʵÄÁżĹ¨¶ČĘÇŁşc(H2SO4)=![]() =18.4mol/LŁ¬ąĘ´đ°¸ÎŞŁş18.4Ł»
=18.4mol/LŁ¬ąĘ´đ°¸ÎŞŁş18.4Ł»
(2).ʵŃéĘŇÎŢ240mLąć¸ńµÄČÝÁżĆżŁ¬ËůŇÔӦѡÓĂ250mLąć¸ńµÄČÝÁżĆż˝řĐĐĹäÖĆŁ¬ÉčĐčҪŨÁňËáµÄĚĺ»ýÎŞa mLŁ¬¸ůľÝϡĘͶ¨ÂÉÓĐŁşa ˇÁ10Ł3LˇÁ18.4mol/L=0.25LˇÁ4.6mol/LŁ¬˝âµĂa=62.5mLŁ»ĹäÖƸĂÁňËáČÜҺʱĐčŇŞµÄŇÇĆ÷Ö÷ŇŞÓĐÁżÍ˛ˇ˘ÉŐ±ˇ˘˛ŁÁ§°ôˇ˘250mLČÝÁżĆżˇ˘˝şÍ·µÎąÜµČŁ¬ąĘ´đ°¸ÎŞŁş62.5Ł»250mLČÝÁżĆżŁ»
(3). ˘Ů. ÁżČˇĹ¨ÁňËáµÄÁżÍ˛ÓĂŐôÁóˮϴµÓ2~3´ÎŁ¬˛˘°ŃĎ´µÓҺתČëČÝÁżĆżÖĐŁ¬»áĘąČÜÖĘĆ«¶ŕŁ¬ĹäÖƵÄČÜҺŨ¶ČĆ«¸ßŁ»
˘Ú.Ňň¶¨ČÝʱҪĽÓË®Ł¬ËůŇÔČÝÁżĆżĘąÓĂʱδ¸ÉÔď¶ÔĹäÖƵÄČÜҺŨ¶ČÎŢÓ°Ď죻
˘Ű.ŨÁňËáϡĘÍ·ĹČČŁ¬ČÜ˝âşóδľŔäČ´ľÍŇĆŇş˛˘¶¨ČÝŁ¬»áĘąČÜŇşµÄĚĺ»ýƫСŁ¬ĹäÖƵÄČÜҺŨ¶ČĆ«¸ßŁ»
˘Ü.¶¨ČÝʱ˛»ĐˇĐÄÓĐÉŮÁżŐôÁóË®µÎµ˝ĆżÍ⣬¶ÔĹäÖƵÄČÜҺŨ¶ČÎŢÓ°Ď죻
˘Ý.¶¨ČÝşóľŐńµ´ˇ˘ŇˇÔȡ˘ľ˛ÖĂŁ¬·˘ĎÖŇşĂćµÍÓڿ̶ČĎߣ¬ÎŞŐýłŁÇéżöŁ¬ČôÔŮĽÓŐôÁóË®˛ąÖÁżĚ¶ČĎß»áĘąČÜŇşĚĺ»ýĆ«´óŁ¬ĹäÖƵÄČÜҺŨ¶ČĆ«µÍŁ¬×ŰÉĎËůĘöŁ¬»áĘąĹäÖƵÄČÜҺŨ¶ČĆ«¸ßµÄĘǢ٢ۣ¬ąĘ´đ°¸ÎŞŁş˘Ů˘ŰˇŁ

 СѧĆÚÄ©±ę׼ĘÔľíϵÁĐ´đ°¸
СѧĆÚÄ©±ę׼ĘÔľíϵÁд𰸡ľĚâÄżˇżĎÖÓĂ0.1000 molˇ¤LŁ1KMnO4ËáĐÔČÜŇşµÎ¶¨Î´ÖŞĹ¨¶ČµÄÎŢÉ«H2C2O4ČÜŇşŁ¬·´Ó¦Ŕë×Ó·˝łĚĘ˝ĘÇŁş2MnO4ŁŁ«5H2C2O4Ł«6H+ = 2Mn2+Ł«10CO2ˇüŁ«8H2O
ĚîżŐÍęłÉÎĘĚ⣺
Ł¨1Ł©¸ĂµÎ¶¨ĘµŃéËůĐčµÄ˛ŁÁ§ŇÇĆ÷ÓĐ______________ˇŁŁ¨Ěî×ÖĸŁ©
AŁ®ËáĘ˝µÎ¶¨ąÜBŁ®ĽîĘ˝µÎ¶¨ąÜ CŁ®ÁżÍ˛ DŁ®×¶ĐÎĆż EŁ®ĚúĽŲ̈FŁ®µÎ¶¨ąÜĽĐGŁ®ÉŐ±HŁ®°×Ö˝ IŁ®Â©¶·
Ł¨2Ł©˛»ÓĂ________(ĚËᡱ»ňˇ°Ľîˇ±)Ę˝µÎ¶¨ąÜʢ·Ĺ¸ßĂĚËáĽŘČÜŇşˇŁĘÔ·ÖÎöÔŇň___________________________________________ˇŁ
Ł¨3Ł©µÎ¶¨ÖŐµăµÄĎÖĎóÎŞ___________________________________ˇŁ
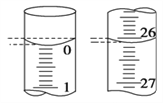
Ł¨4Ł©ČôµÎ¶¨żŞĘĽşÍ˝áĘřʱŁ¬µÎ¶¨ąÜÖеÄŇşĂćČçÍĽËůĘľŁ¬ÔňĆđĘĽ¶ÁĘýÎŞ________mLŁ¬ÖŐµă¶ÁĘýÎŞ________mLˇŁ
Ł¨5Ł©ÄłŃ§Éú¸ůľÝ3´ÎʵŃé·Ö±đĽÇÂĽÓĐąŘĘýľÝČçĎÂ±íŁş
µÎ¶¨ ´ÎĘý | ´ý˛âH2C2O4ČÜŇşµÄĚĺ»ý/mL | 0.1000 mol/L KMnO4µÄĚĺ»ýŁ¨mLŁ© | ||
µÎ¶¨Ç°żĚ¶Č | µÎ¶¨şóżĚ¶Č | ČÜŇşĚĺ»ý/mL | ||
µÚŇ»´Î | 25.00 | 0.00 | 26.11 | 26.11 |
µÚ¶ţ´Î | 25.00 | 1.56 | 30.30 | 28.74 |
µÚČý´Î | 25.00 | 0.22 | 26.31 | 26.09 |
ŇŔľÝÉϱíĘýľÝÁĐĘ˝ĽĆËă¸ĂH2C2O4ČÜŇşµÄÎďÖʵÄÁżĹ¨¶ČÎŞ_______________ˇŁ
Ł¨6Ł©ĎÂÁвŮ×÷ÖĐżÉÄÜĘą˛â¶¨˝áąűĆ«µÍµÄĘÇ___________(Ěî×Öĸ)ˇŁ
AŁ®ËáĘ˝µÎ¶¨ąÜδÓñę׼ҺČóĎ´ľÍÖ±˝ÓעČëKMnO4±ę׼Һ
BŁ®µÎ¶¨Ç°Ę˘·Ĺ˛ÝËáČÜŇşµÄ׶ĐÎĆżÓĂŐôÁóˮϴľ»şóĂ»ÓиÉÔď
CŁ®ËáĘ˝µÎ¶¨ąÜĽâ×첿·ÖÔڵζ¨Ç°Ă»ÓĐĆřĹÝŁ¬µÎ¶¨şóÓĐĆřĹÝ
DŁ®¶ÁȡKMnO4±ę׼ҺʱŁ¬żŞĘĽŃöĘÓ¶ÁĘýŁ¬µÎ¶¨˝áĘřʱ¸©ĘÓ¶ÁĘý
ˇľĚâÄżˇżŇŃÖŞŁşCO(g)Ł«H2O(g)![]() CO2(g)Ł«H2(g)ˇˇ¦¤HŁ˝Ł41 kJˇ¤molŁ1ˇŁĎŕͬζČĎÂŁ¬ÔÚČÝ»ýĎŕͬµÄÁ˝¸öşăÎÂĂܱŐČÝĆ÷ÖĐŁ¬ĽÓČëŇ»¶¨ÁżµÄ·´Ó¦Îď·˘Éú·´Ó¦ˇŁĎŕąŘĘýľÝČçĎÂŁş
CO2(g)Ł«H2(g)ˇˇ¦¤HŁ˝Ł41 kJˇ¤molŁ1ˇŁĎŕͬζČĎÂŁ¬ÔÚČÝ»ýĎŕͬµÄÁ˝¸öşăÎÂĂܱŐČÝĆ÷ÖĐŁ¬ĽÓČëŇ»¶¨ÁżµÄ·´Ó¦Îď·˘Éú·´Ó¦ˇŁĎŕąŘĘýľÝČçĎÂŁş
ČÝĆ÷±ŕşĹ | Ćđʼʱ¸÷ÎďÖĘÎďÖʵÄÁż/mol | ´ďĆ˝şâąýłĚĚĺϵÄÜÁżµÄ±ä»Ż | |||
CO | H2O | CO2 | H2 | ||
˘Ů | 1 | 4 | 0 | 0 | ·ĹłöČČÁżŁş32.8 kJ |
˘Ú | 0 | 0 | 1 | 4 | ČČÁż±ä»ŻŁşQ kJ |
ĎÂÁĐ˵·¨ÖĐŁ¬˛»ŐýČ·µÄĘÇ(ˇˇˇˇ)
A. ČÝĆ÷˘ŮÖĐ·´Ó¦´ďĆ˝şâʱŁ¬COµÄת»ŻÂĘÎŞ80%
B. ČÝĆ÷˘ŮÖĐCOµÄת»ŻÂʵČÓÚČÝĆ÷˘ÚÖĐCO2µÄת»ŻÂĘ
C. Ć˝şâʱŁ¬Á˝ČÝĆ÷ÖĐCO2µÄŨ¶ČĎŕµČ
D. ČÝĆ÷˘ŮʱCOµÄ·´Ó¦ËŮÂʵČÓÚH2OµÄ·´Ó¦ËŮÂĘ
ˇľĚâÄżˇżÔÚ100ˇćʱŁ¬˝«0.100mol N2O4ĆřĚĺłäČë1LşăČÝłéżŐµÄĂܱŐČÝĆ÷ÖĐŁ¬·˘Éú·´Ó¦ŁşN2O4Ł¨gŁ©![]() 2NO2Ł¨gŁ©ˇŁ¸ôŇ»¶¨Ę±Ľä¶Ô¸ĂČÝĆ÷ÄÚÎďÖʵÄŨ¶Č˝řĐĐ·ÖÎöµĂµ˝Čç±íĘýľÝˇŁ»Ř´đÓĐąŘÎĘĚâ;
2NO2Ł¨gŁ©ˇŁ¸ôŇ»¶¨Ę±Ľä¶Ô¸ĂČÝĆ÷ÄÚÎďÖʵÄŨ¶Č˝řĐĐ·ÖÎöµĂµ˝Čç±íĘýľÝˇŁ»Ř´đÓĐąŘÎĘĚâ;
ʱĽä(s) | 0 | 20 | 40 | 60 | 80 |
c(N2O4)/molˇ¤LŁ1 | 0.100 | c1 | 0.050 | c3 | c4 |
c(NO2)/molˇ¤LŁ1 | 0.000 | 0.060 | c2 | 0.120 | 0.120 |
˘Ůc3________c4(Ě>ˇ±ˇ˘ˇ°<ˇ±»ňˇ°Ł˝ˇ±)ˇŁ
˘ÚÔÚÉĎĘöĚőĽţĎÂŁ¬´Ó·´Ó¦żŞĘĽÖ±ÖÁ´ďµ˝»ŻŃ§Ć˝şâʱŁ¬N2O4µÄĆ˝ľů·´Ó¦ËŮÂĘÎŞ_________molˇ¤LŁ1ˇ¤sŁ1ˇŁ
˘Ű´ďĆ˝şâşóĎÂÁĐĚőĽţµÄ¸Ä±äżÉĘąNO2ĆřĚĺŨ¶ČÔö´óµÄĘÇ_______(ĚîĐňşĹ)ˇŁ
AŁ®Ŕ©´óČÝĆ÷µÄČÝ»ý BŁ®ÔŮłäČëŇ»¶¨ÁżµÄN2O4
CŁ®·ÖŔëłöŇ»¶¨ÁżµÄNO2 DŁ®ÔŮłäČëŇ»¶¨ÁżµÄHe
˘ÜČôÔÚĎŕͬĚőĽţĎÂŁ¬ĆđʼʱֻłäČë0.200 mol NO2ĆřĚ壬Ôň´ďµ˝Ć˝şâʱNO2ĆřĚĺµÄת»ŻÂĘÎŞ_________ˇŁ