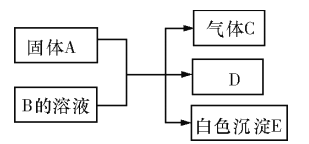
��Ŀ����
����Ŀ����1��18.6gNa2X����0.6molNa+����X�����ԭ������Ϊ___________�������ʵĻ�ѧʽ__________��
��2��2molCO(NH2)2�к�_____mol̼ԭ�ӣ�_____mol��ԭ�ӣ�_____mol��ԭ�ӣ�____mol��ԭ�ӣ�������ԭ������_____molH2O��������ԭ������ȡ�
��3����֪ 1.204��1023 �� X ������ӵ�����Ϊ 8g���� X �����Ħ��������__________��
��4��ͬ��ͬѹ�£�ͬ�����N2��SO2������֮��Ϊ__________________�����ʵ���֮��Ϊ____________��ԭ������֮��Ϊ____________��Ħ������֮��Ϊ__________������֮��Ϊ________________��
���𰸡� 16 Na2O 2 2 4 8 2 40g��mol��1 1:1 1:1 2:3 7:16 7:16
����������1��. ÿ��Na2X�к���2�������ӣ�����0.6mol Na+��Na2X�����ʵ���Ӧ����0.3mol����M(Na2X)= ![]() =
=![]() =62g/mol��Ħ����������ֵ�ϵ�������Է�������������Na2X����Է�������Ϊ62��X���ԭ������=6223��2=16����XΪOԪ���������ʻ�ѧʽΪNa2O���ʴ�Ϊ�� 16��Na2O��
=62g/mol��Ħ����������ֵ�ϵ�������Է�������������Na2X����Է�������Ϊ62��X���ԭ������=6223��2=16����XΪOԪ���������ʻ�ѧʽΪNa2O���ʴ�Ϊ�� 16��Na2O��
��2��. 1��CO(NH2)2�����к�1��̼ԭ�ӡ�1����ԭ�ӡ�2����ԭ�Ӻ�4����ԭ�ӣ����2molCO(NH2)2�к���2mol��̼ԭ�ӡ�2mol��ԭ�ӡ�4mol��ԭ�Ӻ�8mol��ԭ�ӣ�
1��ˮ���Ӻ�1����ԭ�ӣ���2molCO(NH2)2�к�2mol��ԭ�ӣ��ʺ�2mol��ԭ�ӵ�ˮ�����ʵ�����2mol��
���Դ��ǣ�2��2��4��8��2��
��3��.1.204��1023 �� X ������ӵ����ʵ�����n��X��= N��NA = 1.204��1023��6.02��1023 mol��1=0.2mol����X�����Ħ��������M��X��= m��n = 8g��0.2mol=40g��mol��1���ʴ��ǣ�40g��mol��1��
��4��.��ͬ��ͬѹ�£�ͬ��������������ͬ�ķ�����������Ҳ������ͬ�����ʵ�������N2��SO2������֮��Ϊ1:1�����ʵ���֮��Ϊ1:1��ԭ������֮��Ϊ1��2:1��3=2:3��Ħ������֮��Ϊ28g/mol:64g/mol=7:16������֮��Ϊ1��28:1��64=7:16���ʴ�Ϊ��1:1�� 1:1��2:3��7:16��7:16��

����Ŀ����һ��������ܱ������У��������»�ѧ��Ӧ��CO2(g) +H2(g)![]() CO (g) +H2O (g)
CO (g) +H2O (g)
�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
t�� | 700 | 800 | 830 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�ش��������⣺
��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK��__________��
��2����˵���÷�Ӧ�ﵽ��ѧƽ��״̬����________��
A��������ѹǿ���� B�����������c(CO)����
C��v��(H2)��v��(H2O) D��c(CO2)��c(CO)
��3��ij�¶��£�ƽ��Ũ�ȷ�����ʽ�� 3c(CO2)��c(H2)��5c(CO)��c(H2O)�����жϴ�ʱ���¶�Ϊ______�档
��4�� 830��ʱ����1L�ܱ������зֱ�Ͷ��lmolH2��lmolCO2��Ӧ�ﵽ��ѧƽ��ʱ��CO2��ת����Ϊ__________�������¶Ȳ��䣬��ƽ����ϵ���ٳ���1molH2��lmolCO2���´ﵽ��ѧƽ��ʱ��CO2��ƽ��ת����_________(���������С�����䡱)��