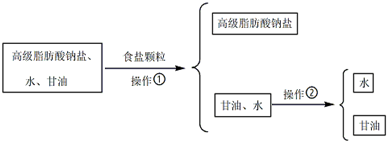
��Ŀ����
6���ձ���ɽ��ѧ���ڱ��ﲩϲ���о�Ա�����µ�ͨ��ʵ�鷢�֣�����Ҷ�ӵ�����ϸ���ܹ������к���ѧ����˫��A�Ķ��ԣ�˫��A�Ľṹ��ʽ��ͼ��ʾ�������йش����ʵ�˵����ȷ���ǣ�������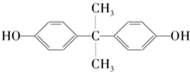
| A�� | 1 mol��������������ˮ��Ӧ����2 mol Br2 | |
| B�� | ����������̼��������Һ��Ӧ�ų�CO2 | |
| C�� | �����ʵ�����̼ԭ�ӿ�����ͬһƽ�� | |
| D�� | ���������������������ӳɷ�Ӧ���������ʵĻ�ѧʽΪC15H28O2 |
���� A�������Ϸ��ǻ���λHԭ���ܱ���ԭ��ȡ����
B�����ǻ���̼�����Ʋ���Ӧ��
C��������������Cԭ�Ӿ��м���ṹ����������Cԭ�Ӳ����ܹ��棻
D�����������������������ӳɷ�Ӧʱ�����ݽṹ��ʽȷ������ʽ��
��� �⣺A�������Ϸ��ǻ���λHԭ���ܱ���ԭ��ȡ����1 mol��������������ˮ��Ӧ����4mol Br2����A����
B�����ǻ���̼�����Ʋ���Ӧ�����Բ������ɶ�����̼����B����
C��������������Cԭ�Ӿ��м���ṹ����������Cԭ�Ӳ����ܹ��棬��C����
D�����������������������ӳɷ�Ӧʱ�����ݽṹ��ʽȷ������ʽΪC15H28O2����D��ȷ��
��ѡD��
���� ���⿼���л���ṹ�����ʣ�Ϊ��Ƶ���㣬��ȷ�����ż������ʹ�ϵ�ǽⱾ��ؼ������ؿ���Ӻͱ������ʣ�ע��Ӻ��巢��ȡ����Ӧʱȡ��λ�ã��״�ѡ����C��

��ϰ��ϵ�д�
�����Ŀ
17��25��ʱ�������йص������Һ�����Ĺ�ϵ��ȷ���ǣ�������
| A�� | pH=4��NaHSO3��Һ�У�c��SO32-����c��H2SO3�� | |
| B�� | pH=3���Ȼ����Һ��pH=3��������ˮ�ĵ���̶���ͬ | |
| C�� | ��pH=5�Ĵ�����Һϡ�ͺ���������Ũ�Ⱦ���С��pH���� | |
| D�� | ��NH4HSO4��Һ�м�������ʵ�����NaOH�γɵ���Һ�У�c��Na+��=c��SO42-����c��NH4+����c��H+����c��OH-�� |
11��ij��Һ����CO32-��HCO3-��S2-��CH3COO-��PO43-���������ӣ���������Һ�м�����������������������Ŀ����ٵ��ǣ�������
| A�� | CO32-��HCO3- | B�� | CO32-��HCO3-��S2- | ||
| C�� | CO32-��HCO3-��S2-��CH3COO- | D�� | ȫ�� |
18���ɶ����ڷǽ���Ԫ��X��Ԫ��Y��ɵĻ�����Y2X3����֪X��ԭ������Ϊn����Y��ԭ�������������ǣ�������
| A�� | n-3 | B�� | n-1 | C�� | n+5 | D�� | n+2 |
15�����б仯��Ҫ��������������ʵ�ֵ��ǣ�������
| A�� | H2��H2O | B�� | H+��H2 | C�� | HClO��HCl | D�� | ClO3-��Cl- |
16����2A��g��+B��g��?3C��g��+4D��g����Ӧ�У���ʾ�÷�Ӧ���������ǣ�������
| A�� | vA=0.05mol/��L•s�� | B�� | vB=0.03mol/��L•s�� | C�� | vC=3.6mol/��L•min�� | D�� | vD=0.1mol/��L•s�� |
 +3NaOH��3C17H35COONa+
+3NaOH��3C17H35COONa+