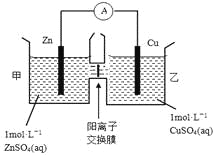
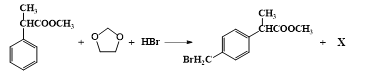
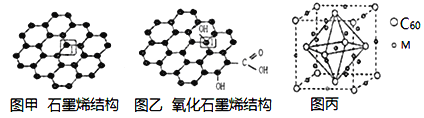
��Ŀ����
����Ŀ��X��Y��Z��M��N��R���ǵ�����������Ԫ�ء�25��ʱ����Ԫ������������Ӧˮ����� pH ��ԭ�Ӱ뾶�Ĺ�ϵ��ͼ������ X��N��W��R �ⶨ����Ũ�Ⱦ�Ϊ0.01 mol/L��Һ��pH��Y��Z�ⶨ�����䱥����Һ��pH������˵����ȷ����

A.R��N�ֱ���X�γɶ�Ԫ�������ˮ��Һ���ʼ���
B.N��Z��X����Ԫ�ص���������������ˮ��Ӧ
C.������ H2���������ѵ�˳���ǣ�R��N��M
D.������������ˮ��Ӧ�����ѵ�˳���ǣ�Y��X��Z
���𰸡�C
��������
X��Y��Z��M��N��R���ǵ�����������Ԫ�أ�25��ʱ����Ԫ������������Ӧˮ�����pH��ԭ�Ӱ뾶�Ĺ�ϵ��ͼ������X��N��W��R�ⶨ����Ũ�Ⱦ�Ϊ0.01mol/L��Һ��pH��Y��Z�ⶨ�����䱥����Һ��pH��X��Y��Z������������Ӧˮ�����ˮ��Һ�ʼ��ԣ���ϼ���ǿ����֪��XΪNa��YΪMg��ZΪAl��R��pH=2����RΪCl��N��pH��2����NΪS��M��pH��4��Ӧ��ΪPԪ�أ��ݴ˽��
���ݷ�����֪��XΪNa��YΪMg��ZΪAl��MΪP��NΪS��RΪClԪ�ء�
A��NaCl��Һ�����ԣ���A����
B��Na��������Ϊ�����ƣ�S�����������Ϊ�����������������ˮ��Ӧ�������ᣬ��������ˮ��Ӧ�����������ƣ���B����
C���ǽ����ԣ�Cl��S��P���ǽ�����Խǿ����������������Խ���ף�������H2���������ѵ�˳���ǣ�R��N��M����C��ȷ��
D�������ԣ�Na��Mg��Al���������������ˮ��Ӧ�����ѵ�˳���ǣ�X��Y��Z����D����
��ѡ��C��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����ȸʯ��һ�ֺ�ͭ��ʯ����ͭ��̬ΪCuCO3��Cu(OH)2��CuSiO3��2H2O��ͬʱ����SiO2��FeCO3��Fe2O3��Al2O3�����ʡ�����Ϊԭ����ȡ����ͭ�Ĺ���������ͼ��
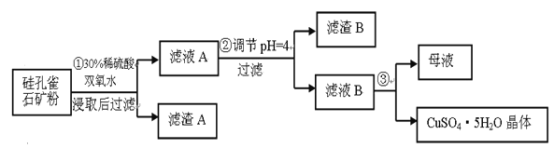
��1����ɲ�������ϡ������CuSiO3��2H2O��Ӧ�Ļ�ѧ����ʽ��
CuSiO3��2H2O+H2SO4=CuSO4+_______+H2O��˫��ˮ��������_________________��
��2��������������ҺpH������ѡ�õ��Լ���______��
A��CuO | B��Fe2O3 | C��Al2O3 | D��Cu(OH)2 |
��3���й��������↑ʼ��������ȫ������pH���±���
�������� | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 |
��ʼ������pH | 3.3 | 1.5 | 6.5 | 4.2 |
������ȫ��pH | 5.2 | 3.7 | 9.7 | 6.7 |
�������У�����pH=4ʱ����������B�ijɷֵĻ�ѧʽΪ_____����ҺB�г�Cu2+��, �����еĽ�����������_______��
��4������ҺBͨ��________��_______�����˵Ȳ����ɵõ�����ͭ���塣
��5���ⶨ����ͭ����ᾧˮ�ĺ���ʱ��Ӧ�������______�����ա�����ʧˮ�����ڿ�������ȴ�������ⶨ���____(����ƫ��������ƫ��������������)��