��Ŀ����
12��A��B��C��D�����ֶ�����Ԫ�أ�E�ǹ���Ԫ�أ�A��B��Cͬ���ڣ�C��Dͬ���壬A��ԭ�ӽṹʾ��ͼΪ��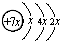 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺��1��д������Ԫ�صķ��ţ�ASi BNa CP DN
��2����Ԫ�ط��ű�ʾD�������ڵ�һ����������Ԫ����F���縺������Ԫ����F��
��3��D���⻯���C���⻯��ķе�ߣ���ߡ��͡�����ԭ����ΪNH3���Ӽ��γ������
��4��EԪ�������ڱ��ĵ������ڢ��壬��֪Ԫ�����ڱ��ɰ������Ų���Ϊs����p����d����ds����f���ȣ���EԪ����d����
��5���õ���ʽ��ʾB��������γɹ��̣�

��6��д��A�������������B������������ˮ���ﷴӦ�����ӷ���ʽSiO2+2OH-=SiO32-+H2O��
���� A��B��C��D�����ֶ�����Ԫ�أ���A��ԭ�ӽṹʾ��ͼ��֪��x=2��A��ԭ������Ϊ14����AΪSiԪ�أ�A��B��Cͬ���ڣ�B��ͬ���ڵ�һ��������С��Ԫ�أ���BΪNaԪ�أ�C��������������ɵ����ӣ���Cԭ�ӵ�3p�ܼ���3�����ӣ���CΪPԪ�أ�C��Dͬ���壬��DΪNԪ�أ�E�ǹ���Ԫ�أ�E����Χ�����Ų�ʽΪ3d64s2��E�ĺ�������Ų�ʽΪ1s22s22p63s23p63d64s2����EΪFeԪ�أ�
��� �⣺A��B��C��D�����ֶ�����Ԫ�أ���A��ԭ�ӽṹʾ��ͼ��֪��x=2��A��ԭ������Ϊ14����AΪSiԪ�أ�A��B��Cͬ���ڣ�B��ͬ���ڵ�һ��������С��Ԫ�أ���BΪNaԪ�أ�C��������������ɵ����ӣ���Cԭ�ӵ�3p�ܼ���3�����ӣ���CΪPԪ�أ�C��Dͬ���壬��DΪNԪ�أ�E�ǹ���Ԫ�أ�E����Χ�����Ų�ʽΪ3d64s2����EΪFeԪ�أ�
��1��������������֪��AΪSi��BΪNa��CΪP��DΪN��
�ʴ�Ϊ��Si��Na��P��N��
��2��ͬ����������ң���һ��������������⣩�����Ե�һ����������Ԫ����F������������ң��縺�����ʵ縺������Ԫ����F��
�ʴ�Ϊ��F��F��
��3����Ϊ�������Ӽ����γ������NH3��PH3�ķе�ߣ�
�ʴ�Ϊ���ߣ���ΪNH3���Ӽ��γ������
��4��EΪFeԪ�أ�Fe�����ڱ��д��ڵ������ڵڢ��壬�������ڱ��д���d ����
�ʴ�Ϊ���ģ�����d��
��5��B������ΪNa2S���õ���ʽ��ʾ�γɹ��̣� ��
��
�ʴ�Ϊ�� ��
��
��6��A�����������ΪSiO2��B������������ˮ����ΪNaOH�����߷�Ӧ�����ӷ���ʽΪ��SiO2+2OH-=SiO32-+H2O��
�ʴ�Ϊ��SiO2+2OH-=SiO32-+H2O��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��ѶȲ����ƶ�Ԫ���ǽ���Ĺؼ���ע����������Ԫ�����������������ʵĵݱ���ɣ�

��H ��O ��Na ��Cl ��Li ��U ��Ca ��K ��Br ��F��
| A�� | �٢ڢۢܢߢ��� | B�� | �ۢܢߢ��� | C�� | �٢ڢۢܢޢߢ��� | D�� | �ۢܢޢߢ��� |
| A�� | ����Ǧ���صĸ�����Pb-2e-�TPb2+ | |
| B�� | п��Ŧ�۵�ص�������Ag2O+2e-+H2O�T2AgOH | |
| C�� | ����п�̵�صĸ�����Zn-2e-+2OH-�TZn��OH��2 | |
| D�� | ��������ȼ�ϵ�ص�������2H2O+4e-�TO2+4H+ |
| A�� | ˮ | B�� | Ũ���� | C�� | ���������Һ | D�� | �� |
| A�� | ͭм��Ũ�����ϼ��� | |
| B�� | ͭм�ڿ��������պ�������ϡH2SO4 | |
| C�� | ͭм��ϡ�����ϼ��� | |
| D�� | ͭм�ڿ��������պ�������ŨH2SO4 |
| A�� | ��й©�����ǿ�����Ժ�ǿ��ʴ�� | |
| B�� | ��Ԫ��λ�ڵ������ڢ�A�� | |
| C�� | ������С�մ���Һ��й©�����г�ϴ | |
| D�� | ʵ���ұ�������ʿ��Խ������ھƾ����л��ܼ��� |
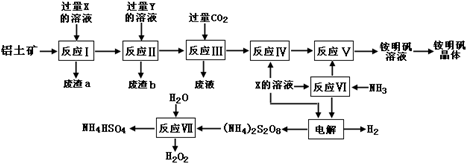
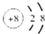
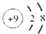
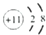

 ��W2Z
��W2Z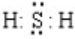 ��
��