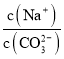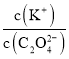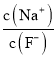��Ŀ����
����Ŀ��������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㡣
��1����֪��CH4(g) + H2O(g)=CO(g) +3H2(g) ��H=+206.2 kJ/mol
CH4(g) + CO2(g)=2CO(g) +2H2(g) ��H=+247.4 kJ/mol
2H2S(g)=2H2(g) +S2(g) ��H=+169.8 kJ/mol
�Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ����CH4(g)��H2O(g)��Ӧ����CO2(g)��H2(g)���Ȼ�ѧ����ʽΪ________________________________��
��2����H2S�ȷֽ�����ʱ������Ӧ����ͨ��һ������������ʹ����H2Sȼ�գ���Ŀ����_________________________________��
��ȼ�����ɵ�SO2��H2S��һ����Ӧ���������ڳ����¾������壬д���÷�Ӧ�Ļ�ѧ����ʽ________________________________��
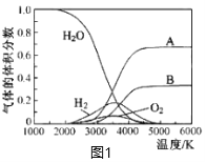
��3��H2O���ȷֽ�Ҳ�ɵõ�H2��������ˮ�ֽ���ϵ����Ҫ���������������¶ȵĹ�ϵ��ͼ1��ʾ��ͼ��A��B��ʾ������������_________________��
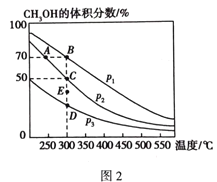
��4����1 molCO��2 molH2��������ܱ������У��ڴ��������·������·�Ӧ��CO(g) + 2H2(g)![]() CH3OH(g)����ͬѹǿ��CH3OH��ƽ���������е�����������¶ȵı仯��ͼ2��ʾ��A��B��C����Ļ�ѧƽ�ⳣ��K(A)��K(B)��K(C)����Դ�СΪ_______________������C���ѹǿƽ�ⳣ��Kp=___________����ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�������������
CH3OH(g)����ͬѹǿ��CH3OH��ƽ���������е�����������¶ȵı仯��ͼ2��ʾ��A��B��C����Ļ�ѧƽ�ⳣ��K(A)��K(B)��K(C)����Դ�СΪ_______________������C���ѹǿƽ�ⳣ��Kp=___________����ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�������������
��5��Mg2Cu��һ�ִ���Ͻ�350 ��ʱ��Mg2Cu��H2��Ӧ������MgCu2�ͽ���һ�ֽ���Ԫ�ص��⻯����������������Ϊ0.077����Mg2Cu��H2��Ӧ�Ļ�ѧ����ʽΪ__________��
���𰸡�CH4(g)+2H2O(g)��CO2(g)+4H2(g) ![]() H��+165.0 kJ��mol-1 ΪH2S�ȷֽⷴӦ�ṩ���� 2H2S+SO2��2H2O+3S��(��4H2S+2SO2��4H2O+3S2��) H��O������ԭ�ӡ���ԭ�ӣ� K(A)��K(B)=K(C)
H��+165.0 kJ��mol-1 ΪH2S�ȷֽⷴӦ�ṩ���� 2H2S+SO2��2H2O+3S��(��4H2S+2SO2��4H2O+3S2��) H��O������ԭ�ӡ���ԭ�ӣ� K(A)��K(B)=K(C) ![]() 2Mg2Cu+3H2
2Mg2Cu+3H2![]() MgCu2+3MgH2
MgCu2+3MgH2
��������
(1)������֪�Ȼ�ѧ����ʽ�������ڸ�˹���ɷ������
(2)ʹ����H2Sȼ�շų�������ΪH2S�ȷֽⷴӦ�ṩ������SO2��H2S��һ����Ӧ���������ڳ����¾������壬���߷�Ӧ����S��H2O��
(3)��ͼ��֪��ˮ�ķֽ⻯ѧ������������Hԭ����Oԭ�ӣ��ݴ˷����жϣ�
(4)�����¶ȶ�ƽ�ⳣ����Ӱ�죬���ͼ������жϣ���������ʽ����ƽ��ʱ�����ʵ����ʵ������ټ����ƽ���ѹ�����ѹǿƽ�ⳣ��Kp�ı���ʽ���㣻
(5)������⻯��ΪRHx�����������������Ϊ0.077�������жϸý����⻯��Ļ�ѧʽ������д����ʽ��
(1)��֪����CH4(g)+H2O(g)��CO(g)+3H2(g)��H=+206.2kJmol-1����CH4(g)+CO2(g)��2CO(g)+2H2(g)��H=+247.4kJmol-1���ɸ�˹���ɣ��١�2-�ڵ�CH4(g)+2H2O(g)��CO2(g)+4H2(g)��H=+165.0 kJmol-1���ʴ�Ϊ��CH4(g)+2H2O(g)��CO2(g)+4H2(g)��H=+165.0 kJmol-1��
(2)��H2S�ȷֽ�����ʱ������Ӧ����ͨ��һ������������ʹ����H2Sȼ�գ��ų�������ΪH2S�ȷֽⷴӦ�ṩ�������ʴ�Ϊ��ΪH2S�ȷֽⷴӦ�ṩ������
��SO2��H2S��һ����Ӧ���������ڳ����¾������壬���߷�Ӧ����S��H2O����Ӧ�Ļ�ѧ����ʽΪ2H2S+SO2��2H2O+3S�����ʴ�Ϊ��2H2S+SO2��2H2O+3S����
(3)��ͼ��֪��ˮ�ķֽ⻯ѧ������������Hԭ����Oԭ�ӣ���ԭ�ӽ��������������ԭ�ӽ��������������ˮ�ķ���ʽ��֪��ԭ�ӵ����ʵ�������ԭ��2������AΪ��ԭ�ӡ�BΪ��ԭ�ӣ��ʴ�Ϊ��AΪ��ԭ�ӡ�BΪ��ԭ�ӣ�
(4)ƽ�ⳣ��Kֻ���¶��йأ�B��C���¶���ͬ����K(B)=K(C)������ͼ�������¶ȣ�CH3OH��ƽ���������е����������С��˵��ƽ�������ƶ����������¶ȣ�K��С�����K(A)��K(B)�����A��B��C����Ļ�ѧƽ�ⳣ��K(A)��K(B)��K(C)����Դ�СΪK(A)��K(B)=K(C)������ͼ��C��ƽ����CH3OH��ƽ���������е��������Ϊ50%���跴Ӧ��CO�����ʵ���Ϊx����
CO(g) + 2H2(g)![]() CH3OH(g)
CH3OH(g)
��ʼ(mol) 1 2 0
��Ӧ(mol) x 2x x
ƽ��(mol) 1-x 2-2x x
��![]() =
=![]() �����x=0.75mol����ƽ���ѹ�ֱ�Ϊ��CO��
�����x=0.75mol����ƽ���ѹ�ֱ�Ϊ��CO��![]() ��p2=
��p2=![]() p2��H2��
p2��H2��![]() ��p2=
��p2=![]() p2��CH3OH��
p2��CH3OH��![]() ��p2=
��p2=![]() p2��ѹǿƽ�ⳣ��Kp=
p2��ѹǿƽ�ⳣ��Kp=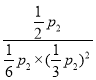 =
=![]() ���ʴ�Ϊ��K(A)��K(B)=K(C)��
���ʴ�Ϊ��K(A)��K(B)=K(C)��![]() ��
��
(5)������⻯��ΪRHx������R����Է�������Ϊa��������Ļ��ϼ�Ϊ+x����Ϊ![]() =0.077����923x=77a����x=2ʱ��a=24���ʸý����⻯��ΪMgH2���ʷ�Ӧ����ʽΪ2Mg2Cu+3H2
=0.077����923x=77a����x=2ʱ��a=24���ʸý����⻯��ΪMgH2���ʷ�Ӧ����ʽΪ2Mg2Cu+3H2![]() MgCu2+3MgH2���ʴ�Ϊ��2Mg2Cu+3H2
MgCu2+3MgH2���ʴ�Ϊ��2Mg2Cu+3H2![]() MgCu2+3MgH2��
MgCu2+3MgH2��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������̼���á��ѳ�Ϊȫ�����ѧ���о�����Ҫ���⣬���ۺ����þ�����Ҫ���塣�ش��������⣺
��1��CO2��CH4�����������Ƶúϳ�����CH4��g��+ CO2��g��![]() 2CO ��g��+ 2H2��g��
2CO ��g��+ 2H2��g��
����֪������Ӧ����صĻ�ѧ�������������£�
��ѧ�� | C��H | C=O | H��H | C |
����/kJ��mol1 | 413 | 745 | 436 | 1075 |
��÷�Ӧ�Ħ�H=_________�����������CH4ƽ��ת���ʵ�������____�����ţ���
A.���µ�ѹ B.���¸�ѹ C.���¸�ѹ D.���µ�ѹ
��ij�¶��£������Ϊ2 L�������м���2 mol CH4��1 mol CO2�Լ���������������Ӧ���ﵽƽ��ʱCO2��ת������50%����ƽ�ⳣ��Ϊ_______mol2��L-2��
��2��CO2���Ա�NaOH��Һ������������Һc��HCO3����c��CO32 -��=2��1����ҺpH=____���������£�H2CO3��K1=4��10-7��K2=5��10-11����0.1mol��L-1 NaHCO3��Һ�������ӵ�Ũ���ɴ�С��˳��Ϊ______________
��3���о�֤ʵ��CO2Ҳ��������ˮ��Һ��ͨ��������ɼ״��������ɼ״��ķ�Ӧ������_______�����õ缫��Ӧʽ��___________________________________