��Ŀ����
����Ŀ���ѿ�ҵ�е����Է�ˮ����Ti��Fe��Ԫ�أ����ۺ�������ͼ![]() ��֪��
��֪��![]() ��ˮ�⣬ֻ�ܴ�����ǿ������Һ��
��ˮ�⣬ֻ�ܴ�����ǿ������Һ��![]() ��
��

(1)����![]() ��Һ�м���
��Һ�м���![]() ��ĩ�ܵõ�����
��ĩ�ܵõ�����![]() ����ԭ����______��
����ԭ����______��
(2)��ȡ![]() �����Ļ�ѧ����ʽΪ______����Ӧ�¶�һ���������
�����Ļ�ѧ����ʽΪ______����Ӧ�¶�һ���������![]() ���£���Ŀ����______��
���£���Ŀ����______��
(3)��֪![]() ����ȡ
����ȡ![]() ʱ��
ʱ��![]() �ﵽ�ܽ�ƽ��ʱ���������²����Һ��pHΪ
�ﵽ�ܽ�ƽ��ʱ���������²����Һ��pHΪ![]() ��
��![]() �����ж����õ�
�����ж����õ�![]() ��______
��______![]() ������������û����
������������û����![]() �������У�Ϊ�˵õ���Ϊ������
�������У�Ϊ�˵õ���Ϊ������![]() �������ʵ����¶��⣬����Ҫ��ȡ�Ĵ�ʩ��______��
�������ʵ����¶��⣬����Ҫ��ȡ�Ĵ�ʩ��______��
(4)Ϊ�˿���![]() ������Ҫ�ⶨ������
������Ҫ�ⶨ������![]() �ĺ�������1g������Ʒ����30mL����ˮ�ܽⲢ����
�ĺ�������1g������Ʒ����30mL����ˮ�ܽⲢ����![]() ��Һ��
��Һ��![]() ��Һ������
��Һ������![]() ����Һ�ζ�����Һ�պñ�ɷۺ�ɫ��ֹͣ�ζ������ı���ҺVmL����Ӧ���漰����Ҫ��ѧ����ʽ�У�
����Һ�ζ�����Һ�պñ�ɷۺ�ɫ��ֹͣ�ζ������ı���ҺVmL����Ӧ���漰����Ҫ��ѧ����ʽ�У�
![]() �Ϻ�
�Ϻ�![]() �ۺ�
�ۺ�![]()
![]() ��
��![]() ��ɫ
��ɫ![]()
��![]() ������______������Ʒ��
������______������Ʒ��![]() �ĺ���Ϊ______
�ĺ���Ϊ______![]() ��
��
���𰸡���Ϊ����ˮ�ⷴӦ��![]() ������
������![]() ��
��![]() ��
��![]() ��Ӧ��
��Ӧ��![]() ��С��ˮ��ƽ������Ӧ�����ƶ�
��С��ˮ��ƽ������Ӧ�����ƶ� ![]() ��ֹ
��ֹ![]() �ֽ���С
�ֽ���С![]() ˮ�� û�� ��Ӧ�����в�����������
ˮ�� û�� ��Ӧ�����в�����������![]() ����
����![]() ����
����![]() ����ɫ����
����ɫ���� ![]()
��������
�ѿ�ҵ�е����Է�ˮ����Ti��Fe��Ԫ�أ�����![]() ��
��![]() ��
��![]() �����ӣ��������ۻ�ԭ�����ӵõ��������ӣ�ͨ������Ũ������ȴ�ᾧ����ϴ�ӡ�����õ������������壬��������̼����泥����˵õ�̼�����������������������յõ������������˺�õ�����
�����ӣ��������ۻ�ԭ�����ӵõ��������ӣ�ͨ������Ũ������ȴ�ᾧ����ϴ�ӡ�����õ������������壬��������̼����泥����˵õ�̼�����������������������յõ������������˺�õ�����![]() ����Һ������
����Һ������![]() ��ĩ��
��ĩ��![]() ��Ӧ����������Һ��
��Ӧ����������Һ��![]() ��ʹƽ��
��ʹƽ��![]() ������
������![]() �ķ����ƶ����ɵõ�
�ķ����ƶ����ɵõ�![]() �ֲ�Ʒ���Դ˽����⡣
�ֲ�Ʒ���Դ˽����⡣
��������������֪��
![]() ������
������![]() ��Һ������
��Һ������![]() ��ĩ��
��ĩ��![]() ��Ӧ����������Һ��
��Ӧ����������Һ��![]() ��ʹƽ��
��ʹƽ��![]() ������
������![]() �ķ����ƶ����ɵõ�
�ķ����ƶ����ɵõ�![]() �ֲ�Ʒ��
�ֲ�Ʒ��
�ʴ�Ϊ����Ϊ����ˮ�ⷴӦ��![]() ������
������![]() ��
��![]() ��
��![]() ��Ӧ��
��Ӧ��![]() ��С��ˮ��ƽ������Ӧ�����ƶ���
��С��ˮ��ƽ������Ӧ�����ƶ���![]() ����������̼����立�Ӧ����̼����������������李�������̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��
����������̼����立�Ӧ����̼����������������李�������̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��![]() ����Ӧ�¶�һ���������
����Ӧ�¶�һ���������![]() ���£���Ϊ�˱����¶ȹ���̼����立ֽ⣬���������ӵ�ˮ��̶ȣ�
���£���Ϊ�˱����¶ȹ���̼����立ֽ⣬���������ӵ�ˮ��̶ȣ�
�ʴ�Ϊ��![]() ����ֹ
����ֹ![]() �ֽ���С
�ֽ���С![]() ˮ�⣻
ˮ�⣻![]() �����²����Һ��pHΪ
�����²����Һ��pHΪ![]() ��
��![]() �����������ݿ�֪����Һ��
�����������ݿ�֪����Һ��![]() ������
������![]() �������ɣ�������У�Ϊ�˵õ���Ϊ������
�������ɣ�������У�Ϊ�˵õ���Ϊ������![]() �������ʵ����¶��⣬����Ҫ��ȡ�Ĵ�ʩ����Ӧ�����в�����������
�������ʵ����¶��⣬����Ҫ��ȡ�Ĵ�ʩ����Ӧ�����в�����������![]() ����
����![]() ��
��
�ʴ�Ϊ��û�У���Ӧ�����в�����������![]() ����
����![]() ��
��![]() ��������ɫ�ʻ�ɫ�������յ��жϣ���
��������ɫ�ʻ�ɫ�������յ��жϣ���![]() ��ɫ
��ɫ![]() ��ɫ
��ɫ![]() �������������Ӹ��ţ�
�������������Ӹ��ţ�
�ʴ�Ϊ������![]() ����ɫ���ţ�
����ɫ���ţ�![]() ����Һ���ĵĸ�����ص����ʵ���Ϊ��
����Һ���ĵĸ�����ص����ʵ���Ϊ��![]() ��
��
���ݷ�Ӧ��![]() ���������������ʵ���Ϊ��
���������������ʵ���Ϊ��![]() ��
��
������Ʒ�к��е�![]() ������Ϊ��
��������![]() ��
��![]() ������������
������������![]() ��
��
�ʴ�Ϊ��![]() ��
��

 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�����Ŀ��ij��ȤС���Է���м�Ƶ���������狀����������Ʊ���ˮ�ϲ�������(FeC2O4��2H2O)����һ���Ʊ��ߴ��Ȼ�ԭ���ۡ�
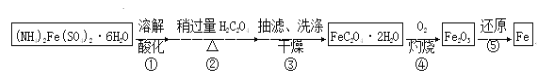
��֪��FeC2O4��2H2O������ˮ��150�濪ʼʧ�ᾧˮ��H2C2O4������ˮ���ܽ�����¶����߶�����
��ش�
��1�����в�����������ȷ����________��
A������ڣ�H2C2O4�Թ�����Ҫ��Ϊ������Fe2+ˮ��
B������ۣ�������ˮϴ�ӿ���߳���Ч��
C������ۣ�ĸҺ�е�������Ҫ��(NH4)2SO4��H2C2O4
D������ۣ�����ڳ�ѹ�¿��ٸ���¶ȿ�ѡ���Ը���100��
��2����ͼװ�ã�����һϵ�в�����ɲ�����еij��˺�ϴ�ӡ���ѡ����ʵı�ţ�����ȷ�IJ���˳������(ϴ�Ӳ���ֻ�迼��һ��)��

�������á�a��b��d��________��c���س����á�
a��ת�ƹ�Һ����b���ػ���A��c��������A��d��ȷ�ϳ�ɣ�e����ϴ�Ӽ�ϴ��
���˺���ͨ������ȣ��ŵ���___________________________________________��
��3�� ��ȡһ������FeC2O4��2H2O�������������ܽ⣬
����KMnO4�ζ����ⶨ�����������£�
n(Fe2+)/mol | n( | ������FeC2O4��2H2O���������� |
9.80��10��4 | 9.80��10��4 | 0.980 |
�ɱ��������Ʋ�����������Ҫ��������____________________��
��4��ʵ�ֲ���ܱ����õ�������������____________________
(��ѡ������a���ձ���b��������c��������ƿ��d������¯��e��������f����ƿ)��
�ò���Ļ�ѧ����ʽ��______________________________________��
��5��Ϊʵ�ֲ���ݣ�������̼�ۻ�ԭFe2O3��������________________________________��