��Ŀ����
A��B��C��D��ԭ������������������ֶ�����Ԫ�أ�BԪ�ص�ԭ�������������Ǵ�����������������AԪ����CԪ�ص�ԭ�ӵĵ��Ӳ������1����CԪ�ص���������븺�۵ľ���ֵ���2��D�ǵؿ��к������ķǽ���Ԫ�ء�������������⣺
��1����A��B��C��D����Ԫ����ɵ���ʽ�Σ��ڼ��������·����ķ�ӦΪ____________��
��2������A�͵���C��һ���������¿����ɻ�����E���÷�Ӧ�Ļ�ѧ����ʽΪ________________________��E����Ŀռ乹��Ϊ____________��
��3��4 g B������������D��������ȫȼ�շ���131.17 kJ,��д���÷�Ӧ���Ȼ�ѧ����ʽ��____________________________________��
��4����һ�����¶��£���һ�����Ϊ2 L���ܱ�������ͨ��2 mol����A��8 mol C���ʣ�3 minʱ�ﵽƽ�⣬���E��Ũ��Ϊ1 mol��L-1����ʱ����C�Ļ�ѧ��Ӧ����Ϊ____________��
��5��������CD��D2���ʷ����ķ�ӦΪ��2CD��g��+D2(g) ![]() 2CD2(g),����һ���¶��£���һ���̶�������ܱ������У�����2 mol CD(g)��1 mol D2(g)����һ�������´��ƽ�⣬���CD��ת����Ϊ80%���������¶Ȳ��䣩����ʱ�������ڵ�ѹǿ����ʼѹǿ֮��С��_____________�����ֵ����
2CD2(g),����һ���¶��£���һ���̶�������ܱ������У�����2 mol CD(g)��1 mol D2(g)����һ�������´��ƽ�⣬���CD��ת����Ϊ80%���������¶Ȳ��䣩����ʱ�������ڵ�ѹǿ����ʼѹǿ֮��С��_____________�����ֵ����
(1)NH4HCO3====NH3��+CO2��+H2O
![]() ������
������
(3)C(s)+O2(g)====CO2(g);��H=-393.51 kJ��mol-1
(4)0.5 mol��L-1��min-1
(5)11/15
����:D�ǵؿ��к������ķǽ���Ԫ�أ���DΪ��Ԫ�أ���Ԫ�ص�ԭ�������������Ǵ�������������������BΪ̼Ԫ�أ�CԪ�ص���������븺�۵ľ���ֵ���2��CΪNԪ�أ���A��B��C��DΪԭ����������������A��C��ԭ�ӵĵ��Ӳ������1��֪AΪHԪ�ء�

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�| A��Ԫ��C�γɵĵ��ʿ����ڵ�ȼ�����ֱ���Ԫ��A��B��D�γɵĵ��ʻ��ϣ����û���������ڹ��ۼ� | B��Ԫ��B��C��D��ԭ�Ӱ뾶�ɴ�С��˳��Ϊ��r��D����r��C����r��B�� | C��1.0 L 0.1 mol/L����Һ�к��������ܵ����ʵ���С��0.1 mol | D��1 mol��������������ȫ��Ӧ��ת��Լ1.204��1024������ |
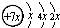 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺

 ��b��c�γɻ�����ĵ���ʽΪ
��b��c�γɻ�����ĵ���ʽΪ �����бȽ�����ȷ���ǣ�������
�����бȽ�����ȷ���ǣ�������