��Ŀ����
����Ŀ��I����1�����������������Ҫԭ���Ǹ��������ƹ����л���������̼���ʣ��ڳ�ʪ�Ŀ����������γ�ԭ���,�����绯ѧ��ʴ�������Ի����£���������ӦʽΪ_____________�������Ժ��������������£��䷢��___________________ (�������ⸯʴ������������ʴ��)��
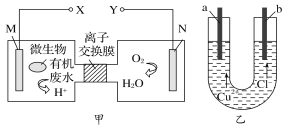
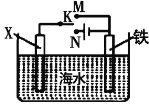
��2��������ͼװ�ã�����ģ�����ĵ绯ѧ��������XΪ̼��������K����N�����õ绯ѧ��������Ϊ____________________����XΪп��������K����M����________��������������������)�ﵽ��ֹ����ʴ![]() Ŀ�ġ�
Ŀ�ġ�
II����ͼ��ʾ�����м׳ص��ܷ�ӦʽΪ2CH3OH+3O2+4KOH=2K2CO3+6H2O�������������:

��1���׳�ȼ�ϵ�صĸ�����ӦΪ___________________��
��2���ҳ���ʯī�缫Ϊ___________��������_____________��Ӧ������������������ԭ����д���ҳ��е���ܷ�Ӧ�Ļ�ѧ����ʽ: ___________________��
��3���׳�������224mL(��״����)O2����ʱ������������������________g��������ʱ�ҳ�����Һ�����Ϊ400mL������Һ��pH=____________��
���𰸡�2H++2e![]() = H2�� ������ʴ ��ӵ�Դ������������������ �� CH3OH- 6e
= H2�� ������ʴ ��ӵ�Դ������������������ �� CH3OH- 6e![]() +8 OH
+8 OH![]() = CO32- +6H2O �� ���� 2CuSO4+ 2H2O
= CO32- +6H2O �� ���� 2CuSO4+ 2H2O ![]() 2Cu+ O2��+2H2SO4 1.16 1
2Cu+ O2��+2H2SO4 1.16 1
��������
I.��1�����ݽ����绯ѧ��ʴ��Ϊ������ʴ�����ⸯʴ���з�����
��2���ӽ��������ķ����Ͻ��з�����
II.��ԭ��ع���ԭ���͵��ԭ���ĽǶȽ��з����ͽ��
I.��1�������绯ѧ��ʴ��Ϊ������ʴ�����ⸯʴ����������Ϊ���ԣ��������ⸯʴ������ԭ��ع���ԭ����������ӦʽΪ2H����2e��=H2������������Ϊ���Ի��������ԣ�����������ʴ������ԭ��صĹ���ԭ����������ӦʽΪO2��4e����2H2O=4OH����
�ʴ�Ϊ2H����2e��=H2����������ʴ��
��2��XΪ̼��������K����N������װ��������ӵ�Դ��װ�����ڵ���ʣ����ݵ��صĹ���ԭ�������缫���������������뷴Ӧ���������������ӵ�����������������XΪп��������K����M������װ��Ϊԭ���װ�ã�п�������ã�пΪ��������Ϊ�����������������÷���������������������������
�ʴ�Ϊ����ӵ����������������ܣ�
II.��1���׳�Ϊ��أ�ͨȼ��һ��Ϊ��������ͨCH3OHһ��Ϊ������ͨ�����������һ��Ϊ�����������ΪKOH��Һ����˸�����ӦʽΪCH3OH��8OH����6e��=CO32����6H2O��
�ʴ�ΪCH3OH��8OH����6e��=CO32����6H2O��
��2���ҳ���ʯī���Ӽ׳�ͨ����һ�������ҳ���ʯīΪ�������ҳ���Ag�����������ݵ��ԭ����������ʧ���ӣ�����������Ӧ��������ӦʽΪ2H2O��4e��=O2����4H���������ϵõ����ӣ�������ԭ��Ӧ��������ӦʽΪCu2����2e��=Cu������ܵ缫��ӦʽΪ2CuSO4��2H2O ![]() 2Cu��O2����2H2SO4��
2Cu��O2����2H2SO4��
�ʴ�Ϊ������������2CuSO4��2H2O ![]() 2Cu��O2����2H2SO4��
2Cu��O2����2H2SO4��
��3�������ܷ�ӦʽΪMgCl2��2H2O![]() Mg(OH)2��H2����Cl2������·Ϊ������·������ת�Ƶ������ʵ�����ͬ�������Ĺ�ϵʽ��Ϊ
Mg(OH)2��H2����Cl2������·Ϊ������·������ת�Ƶ������ʵ�����ͬ�������Ĺ�ϵʽ��Ϊ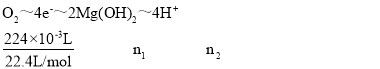 ���n1=0.02mol��������Mg(OH)2����Ϊ0.02mol��58g��mol��1=1.16g��n2=0.04mol���ҳ���c(H��)=
���n1=0.02mol��������Mg(OH)2����Ϊ0.02mol��58g��mol��1=1.16g��n2=0.04mol���ҳ���c(H��)=![]() =0.1mol��L��1����pH=1��
=0.1mol��L��1����pH=1��
�ʴ�Ϊ1.16g��1��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����������Ҫ����ԭ�ϣ��ڹ�����ռ��Ҫ��λ����ҵ�ϳɰ��ķ�ӦΪ��N2(g)+3H2(g) 2NH3(g) ��H��0
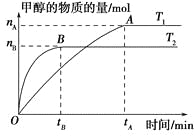
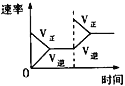
��1��ͼ��ʾ�ϳ�NH3��Ӧ��ij��ʱ��t0��t6�з�Ӧ�����뷴Ӧ����![]() ����ͼ��t1��t3��t4 ʱ�̷ֱ�ı�ijһ����������������е��ﻯѧƽ���ʱ����У�NH3�����������С��һ��ʱ����___________(��д�������)
����ͼ��t1��t3��t4 ʱ�̷ֱ�ı�ijһ����������������е��ﻯѧƽ���ʱ����У�NH3�����������С��һ��ʱ����___________(��д�������)
A��t0��t1 B��t2��t3 C��t3��t4 D��t5��t6
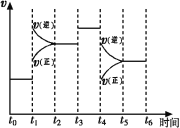
t4ʱ�ı��������________________��
�ֽ��������о�:��773Kʱ���ֱ�2molN2��6molH2����һ���̶��ݻ�Ϊ1L���ܱ������У����ŷ�Ӧ�Ľ��У�����������n(H2)��n(NH3)�뷴Ӧʱ��t�Ĺ�ϵ�����
t/min | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
n(H2)/mol | 6.00 | 4.50 | 3.60 | 3.30 | 3.03 | 3.00 | 3.00 |
n(NH3)/mol | 0 | 1.00 | 1.60 | 1.80 | 1.98 | 2.00 | 2.00 |
��2����Ӧ��0��10�������Ե���Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ___________�����¶��£��˷�Ӧ��ƽ�ⳣ��K=____________��
��3�����¶��£�����ͬ�ݻ�����һ������Ͷ���N2��H2��NH3��Ũ�ȷֱ�Ϊ3mo/L��3mol/L��3mo/L/span>�����ʱV��_____V�� (����>����<������=��)��
��4�����ϱ��е�ʵ�����ݼ���õ���Ũ��һʱ�����Ĺ�ϵ������ͼ�е����߱�ʾ����ʾc(N2)-t��������______�����������������������������ڴ��¶��£�����ʼ����4molN2��12 molH2����Ӧ�մﵽƽ��ʱ����ʾc(H2)-t����������Ӧ�ĵ�Ϊ_________��
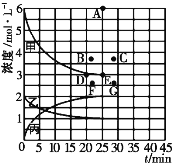
����Ŀ������ʵ���������Ӧ�������Լ����۶���ȷ�����߾��������ϵ����
ѡ�� | ʵ�� | ���� | ���� |
A | ��1mL 0.1mol/L AgNO3��Һ�е���2��0.1 mol/L ��NaCl��Һ�����������Һ�е���2��0.1 mol/L KI��Һ������� | �Ȳ�����ɫ����������ֻ�ɫ���� | Ksp(AgCl) >Ksp (AgI) |
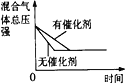
B | һ�������£��ܱ�������Ӧ��ƽ�⣺ H2(g)+I2(g) | ������ɫ���� | ƽ������ |
C | ���������pH��HA��HB��������Һ�ֱ��������Ĵ�С��ͬ��п��Ӧ | ��Ӧ��ʼ��HA����H2�����ʸ��� | HA������ |
D | ��п����ϡ���ᷴӦ���Թ��еμӼ���CuSO4��Һ | ����������������Լӿ� | CuSO4�Ը÷�Ӧ�д����� |
A.AB.BC.CD.D