��Ŀ����
(1)�뽫�����������ʣ�KBr��Br2��I2��KI��K2SO4�ֱ��������к����ϣ����һ��δ��ƽ�Ļ�ѧ����ʽ��
KBrO3�� ��H2SO4���� �� �� �� ��H2O��
(2)����û�ѧ����ʽ��I2��KBr�Ļ�ѧ�������ֱ���8��1�����Br2�Ļ�ѧ�������� ��
���뽫��Ӧ��Ļ�ѧʽ����ƽ��Ļ�ѧ����������������Ӧ��λ���У�
KBrO3�� �� H2SO4����������
����ת��10 mol���ӣ���Ӧ������I2�����ʵ���Ϊ ��
KBrO3�� ��H2SO4���� �� �� �� ��H2O��
(2)����û�ѧ����ʽ��I2��KBr�Ļ�ѧ�������ֱ���8��1�����Br2�Ļ�ѧ�������� ��
���뽫��Ӧ��Ļ�ѧʽ����ƽ��Ļ�ѧ����������������Ӧ��λ���У�
KBrO3�� �� H2SO4����������
����ת��10 mol���ӣ���Ӧ������I2�����ʵ���Ϊ ��
(1)KI I2 Br2 K2SO4 KBr
(2)��1 ��3 16KI 9 ��5 mol
(2)��1 ��3 16KI 9 ��5 mol
(1)����KBrO3�ڷ�Ӧ��BrԪ�صĻ��ϼ۽��ͣ�֪�������������������뻹ԭ��KI�������õ�δ��ƽ�Ļ�ѧ����ʽΪKBrO3��KI��H2SO4����I2��Br2��K2SO4��KBr��H2O��
(2)�����I2�Ļ�ѧ��������8��KBr�Ļ�ѧ��������1������ݵ�Ԫ�ػ��ϼ۱仯֪��ʧ����16 mol��KBr�Ļ�ѧ��������1���õ���Ϊ6 mol����KBrO3��Br2���õ���10 mol����Br2�Ļ�ѧ������Ϊ1�����ɢ��б仯��֪��KI�Ļ�ѧ������Ϊ16��KBrO3�Ļ�ѧ������Ϊ3���ٸ���Kԭ���غ��Ƴ�K2SO4�Ļ�ѧ������Ϊ9������H2SO4�Ļ�ѧ������Ϊ9����3KBrO3��16KI��9H2SO4=8I2��Br2��9K2SO4��KBr��9H2O������ת��10 mol���ӣ�
��16KI �� 16e�� �� 8I2
10 y
���y��5 mol��
(2)�����I2�Ļ�ѧ��������8��KBr�Ļ�ѧ��������1������ݵ�Ԫ�ػ��ϼ۱仯֪��ʧ����16 mol��KBr�Ļ�ѧ��������1���õ���Ϊ6 mol����KBrO3��Br2���õ���10 mol����Br2�Ļ�ѧ������Ϊ1�����ɢ��б仯��֪��KI�Ļ�ѧ������Ϊ16��KBrO3�Ļ�ѧ������Ϊ3���ٸ���Kԭ���غ��Ƴ�K2SO4�Ļ�ѧ������Ϊ9������H2SO4�Ļ�ѧ������Ϊ9����3KBrO3��16KI��9H2SO4=8I2��Br2��9K2SO4��KBr��9H2O������ת��10 mol���ӣ�
��16KI �� 16e�� �� 8I2
10 y
���y��5 mol��

��ϰ��ϵ�д�
�����Ŀ

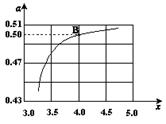
 Cu2O��H2������ʯīӦ���Դ��________��������ͭ�缫�ϵĵ缫��ӦʽΪ________���������У���������Χ��ҺpH________(��������С�����䡱)��
Cu2O��H2������ʯīӦ���Դ��________��������ͭ�缫�ϵĵ缫��ӦʽΪ________���������У���������Χ��ҺpH________(��������С�����䡱)��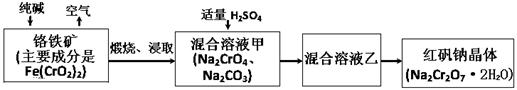
 2CrO42-+2H+����д����ƽ���ƽ�ⳣ������ʽK= ����������ˮϡ�ͣ�ƽ�⽫ �ƶ�(���������������)��
2CrO42-+2H+����д����ƽ���ƽ�ⳣ������ʽK= ����������ˮϡ�ͣ�ƽ�⽫ �ƶ�(���������������)�� (NxOy)�� H2O(��ƽʱx��y�þ�����ֵ��ʾ����������
(NxOy)�� H2O(��ƽʱx��y�þ�����ֵ��ʾ���������� CO(NH2)2 (l��+ H2O (l)��
CO(NH2)2 (l��+ H2O (l)��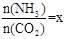 ����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ���� ��
����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ���� ��