��Ŀ����
��¯����ʱ�ô���������������Ʒ�����ݲ��������ȷ���л������ɣ���ͼװ������ȼ�շ�ȷ���л������ʽ�ij���װ�á�
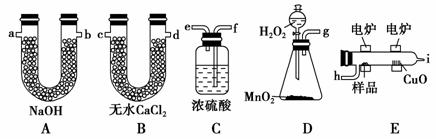
(1)�����������������ҷ�����������ѡװ�ø����ܵ�����˳����________________________________________________________________��
(2)Cװ����Ũ�����������__________________________________________
______________________________��
(3)Dװ����MnO2��������__________________________________________
_____________________________��
(4)ȼ�չ���CuO��������___________________________________________
____________________________��
(5)��ȷ��ȡ0.90 g��Ʒ(ֻ��C��H��O����Ԫ���е����ֻ�����)�������ȼ�պ�A����������1.32 g��B����������0.54 g������л����ʵ��ʽΪ________��
(6)Ҫȷ�����л���ķ���ʽ����Ҫ֪��______________________________
_________________________________________��
������(1)Ҫȷ���л���ķ���ʽ������Ҫȷ�������Ԫ�ء��������Ϣ��֪��Bװ������ˮ�֣�Aװ������CO2��Bװ��Ҫ����Aװ��ǰ�棬����Aװ�ûὫCO2��H2Oһͬ���ա��л����ڵ�¯��ȼ����ҪO2������ҪDװ�����¯������Dװ���ṩ��O2����ˮ�֣����Ӧ����Cװ�ó�ˮ�֣��ʸ����ܵ�����˳���� g��f��e��h��i��c(��d)��d(��c)��a(��b)��b(��a)��
(2)Cװ����Ũ������������O2��
(3)Dװ����MnO2��H2O2�ֽ����������������ã��ӿ�����O2�����ʡ�
(4)��CuOʹ�л�������������CO2��H2O��
(5)�и������ݿ�֪��n(C)��n(CO2)�� ��0.03 mol��n(H)��2n(H2O)��2��
��0.03 mol��n(H)��2n(H2O)��2��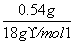 ��0.06 mol��
��0.06 mol��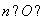
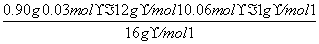 ��0.03 mol��
��0.03 mol��
��N(C)��N(H)��N(O)��n(C)��n(H)��n(O)��0.03 mol��0.06 mol��0.03 mol��1��2��1�����Ը��л����ʵ��ʽΪCH2O��
(6)��(5)�ѵõ����л�����ӵ�ʵ��ʽΪCH2O��ֻҪ��֪�����л�����ӵ���Է����������Ϳ���������ʽ���������ʽΪ(CH2O)n��
��n��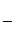 ��
��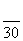 ��
��
�𰸡�(1)g��f��e��h��i��c(��d)��d(��c)��a(��b)��b(��a)��(2)����ˮ�֣��õ������O2��(3)�������ӿ�����O2�����ʡ�(4)ʹ�л�������������CO2��H2O��(5)CH2O
(6)�л������Է�������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�N2O5��һ����������������һ���¶��¿ɷ������·�Ӧ��
2N2O5(g)  4NO2 (g)+ O2(g) ��H > 0��t��ʱ�����ܱ�������ͨ��N2O5������ʵ�����ݼ��±���
4NO2 (g)+ O2(g) ��H > 0��t��ʱ�����ܱ�������ͨ��N2O5������ʵ�����ݼ��±���
| ʱ��/s | 0 | 500 | 1000 | 1500 |
| c(N2O5)/ mol��L-1 | 5.00 | 3.52 | 2.50 | 2.50 |
����˵��������ȷ���� ( )
A��500 s ��N2O5�ֽ�����Ϊ2.96��10-3mol��L-1��s-1
B����ƽ��ʱ��N2O5��ת����Ϊ50%
C����ƽ��������¶�ƽ�ⳣ����С
D����ƽ��������������䣬���������ѹ����ԭ����һ�룬c(N2O5)> 5.00 mol��L-1
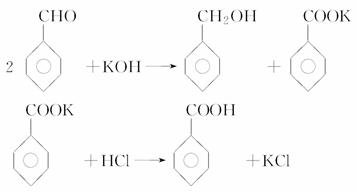
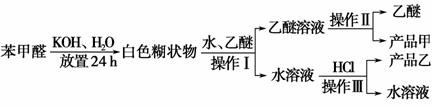
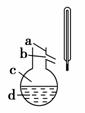 (4)����ͼ��ʾ�����������¶ȼ�ˮ�������ط��õ�λ��Ӧ��________(�a������b������c����d��)���ò����У�����������ƿ���¶ȼ��⣬����Ҫ�IJ���������________________���ռ���Ʒ�������¶�Ϊ________��
(4)����ͼ��ʾ�����������¶ȼ�ˮ�������ط��õ�λ��Ӧ��________(�a������b������c����d��)���ò����У�����������ƿ���¶ȼ��⣬����Ҫ�IJ���������________________���ռ���Ʒ�������¶�Ϊ________��