��Ŀ����
��������(NaClO2)��һ��ǿ������Ư�����㷺���ڷ�֯��ӡȾ��ʳƷ��ҵ�����ڼ��Ի������ȶ����ڡ�ijͬѧ�������Ϻ��������NaClO2����Ҫ�������¡�

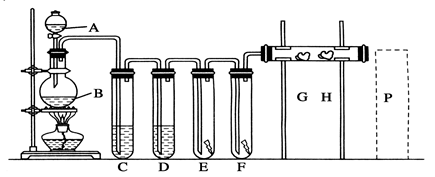
(1)˫��ˮ�ĽṹʽΪ____________�����з�����Ӧ�Ļ�ԭ����__________(�ѧʽ)��
(2)���з�Ӧ�����ӷ���ʽ��_______________________________________��
(3)A�Ļ�ѧʽ��________��װ�â���A��________����������
(4)ClO2��һ�ָ�Чˮ�������������������ƺ�ϡ����Ϊԭ���Ʊ���
��д���÷�Ӧ�Ļ�ѧ����ʽ��__________________________________________��
���о�����������Ӧ��ʼʱ����Ũ�Ƚϴ��������������Cl2�������ӷ���ʽ���Ͳ���Cl2��ԭ��__________________________________________��
(5)NaClO2���ʿɷֽ�ΪNaClO3��NaCl��ȡ������NaClO2������һ�ݸ����ʵ�����ʹ֮���ʣ���һ���ϸ棬�������Һ�����ֱ�������FeSO4��Һ��Ӧʱ������Fe2�������ʵ���________(���ͬ��������ͬ�������жϡ�)��

(1)˫��ˮ�ĽṹʽΪ____________�����з�����Ӧ�Ļ�ԭ����__________(�ѧʽ)��
(2)���з�Ӧ�����ӷ���ʽ��_______________________________________��
(3)A�Ļ�ѧʽ��________��װ�â���A��________����������
(4)ClO2��һ�ָ�Чˮ�������������������ƺ�ϡ����Ϊԭ���Ʊ���
��д���÷�Ӧ�Ļ�ѧ����ʽ��__________________________________________��
���о�����������Ӧ��ʼʱ����Ũ�Ƚϴ��������������Cl2�������ӷ���ʽ���Ͳ���Cl2��ԭ��__________________________________________��
(5)NaClO2���ʿɷֽ�ΪNaClO3��NaCl��ȡ������NaClO2������һ�ݸ����ʵ�����ʹ֮���ʣ���һ���ϸ棬�������Һ�����ֱ�������FeSO4��Һ��Ӧʱ������Fe2�������ʵ���________(���ͬ��������ͬ�������жϡ�)��
(1)H��O��O��H��Na2SO3
(2)2ClO2 ��H2O2��2OH��=2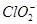 ��O2����2H2O
��O2����2H2O
(3)H2SO4����
(4)5NaClO2��4HCl=5NaCl��4ClO2����2H2O
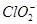 ��3Cl����4H��=2Cl2����2H2O
��3Cl����4H��=2Cl2����2H2O
(5)��ͬ
(2)2ClO2 ��H2O2��2OH��=2
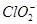 ��O2����2H2O
��O2����2H2O(3)H2SO4����
(4)5NaClO2��4HCl=5NaCl��4ClO2����2H2O
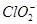 ��3Cl����4H��=2Cl2����2H2O
��3Cl����4H��=2Cl2����2H2O(5)��ͬ
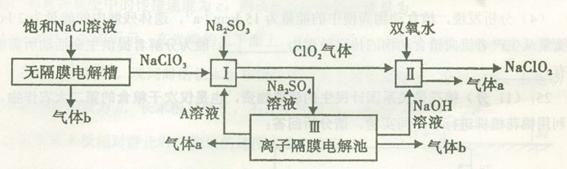
(1)NaClO3��Clԭ�ӻ��ϼ�Ϊ��5�ۣ����к�ǿ�������ԣ���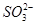 �����������¾��н�ǿ�Ļ�ԭ�ԣ��ʢ��з�����Ӧ����������NaClO3����ԭ����Na2SO3��(2)���Na2SO4��Һ�൱�ڵ��ˮ��������a��b��Na2SO4��Һ�ĵ������֪ΪH2��O2��������aΪ��Ӧ��IJ���֮һ��֪��ΪO2��ΪH2O2��ClO2�����õ��IJ���ʷ�Ӧ��ķ�Ӧ��ΪClO2��H2O2��NaOH����������NaClO2��O2�����ݵ����غ��ԭ���غ���ƽ���ɣ�(3)���Na2SO4��ҺʱOH�����������ɣ���Na��ͨ�����Ӹ�Ĥ�������ƶ��õ�����NaOH��Һ������������H����
�����������¾��н�ǿ�Ļ�ԭ�ԣ��ʢ��з�����Ӧ����������NaClO3����ԭ����Na2SO3��(2)���Na2SO4��Һ�൱�ڵ��ˮ��������a��b��Na2SO4��Һ�ĵ������֪ΪH2��O2��������aΪ��Ӧ��IJ���֮һ��֪��ΪO2��ΪH2O2��ClO2�����õ��IJ���ʷ�Ӧ��ķ�Ӧ��ΪClO2��H2O2��NaOH����������NaClO2��O2�����ݵ����غ��ԭ���غ���ƽ���ɣ�(3)���Na2SO4��ҺʱOH�����������ɣ���Na��ͨ�����Ӹ�Ĥ�������ƶ��õ�����NaOH��Һ������������H����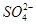 �������ƶ��õ�����A(��H2SO4��Һ)��(4)���������֪��Ӧ��ΪNaClO2��HCl������NaClO2��ClԪ�صĻ��ϼ�Ϊ��3�ۣ��ȿ�����Ҳ�ɽ��ͣ��ʷ�ӦΪNaClO2������������ԭ��Ӧ�����ݵ����غ��֪��������ClO2�ͻ�ԭ����Cl�������ʵ���֮��Ϊ4��1������ԭ���غ㽫����ʽ��ƽ���ɣ���������ΪCl2��
�������ƶ��õ�����A(��H2SO4��Һ)��(4)���������֪��Ӧ��ΪNaClO2��HCl������NaClO2��ClԪ�صĻ��ϼ�Ϊ��3�ۣ��ȿ�����Ҳ�ɽ��ͣ��ʷ�ӦΪNaClO2������������ԭ��Ӧ�����ݵ����غ��֪��������ClO2�ͻ�ԭ����Cl�������ʵ���֮��Ϊ4��1������ԭ���غ㽫����ʽ��ƽ���ɣ���������ΪCl2��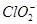 ��HCl�����˹��з�Ӧ�����ݵ����غ��֪��Ӧ��
��HCl�����˹��з�Ӧ�����ݵ����غ��֪��Ӧ��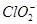 ��HCl�����ʵ���֮��Ϊ3��1������ԭ���غ㽫����ʽ��ƽ���ɣ�(5)���ʹ���ΪNaClO2������������ԭ��Ӧ�����۱��������Fe2����Ӧʱ��Ԫ�����ն�ת��ΪCl������ת�Ƶ�������ȡ�
��HCl�����ʵ���֮��Ϊ3��1������ԭ���غ㽫����ʽ��ƽ���ɣ�(5)���ʹ���ΪNaClO2������������ԭ��Ӧ�����۱��������Fe2����Ӧʱ��Ԫ�����ն�ת��ΪCl������ת�Ƶ�������ȡ�
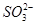 �����������¾��н�ǿ�Ļ�ԭ�ԣ��ʢ��з�����Ӧ����������NaClO3����ԭ����Na2SO3��(2)���Na2SO4��Һ�൱�ڵ��ˮ��������a��b��Na2SO4��Һ�ĵ������֪ΪH2��O2��������aΪ��Ӧ��IJ���֮һ��֪��ΪO2��ΪH2O2��ClO2�����õ��IJ���ʷ�Ӧ��ķ�Ӧ��ΪClO2��H2O2��NaOH����������NaClO2��O2�����ݵ����غ��ԭ���غ���ƽ���ɣ�(3)���Na2SO4��ҺʱOH�����������ɣ���Na��ͨ�����Ӹ�Ĥ�������ƶ��õ�����NaOH��Һ������������H����
�����������¾��н�ǿ�Ļ�ԭ�ԣ��ʢ��з�����Ӧ����������NaClO3����ԭ����Na2SO3��(2)���Na2SO4��Һ�൱�ڵ��ˮ��������a��b��Na2SO4��Һ�ĵ������֪ΪH2��O2��������aΪ��Ӧ��IJ���֮һ��֪��ΪO2��ΪH2O2��ClO2�����õ��IJ���ʷ�Ӧ��ķ�Ӧ��ΪClO2��H2O2��NaOH����������NaClO2��O2�����ݵ����غ��ԭ���غ���ƽ���ɣ�(3)���Na2SO4��ҺʱOH�����������ɣ���Na��ͨ�����Ӹ�Ĥ�������ƶ��õ�����NaOH��Һ������������H����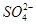 �������ƶ��õ�����A(��H2SO4��Һ)��(4)���������֪��Ӧ��ΪNaClO2��HCl������NaClO2��ClԪ�صĻ��ϼ�Ϊ��3�ۣ��ȿ�����Ҳ�ɽ��ͣ��ʷ�ӦΪNaClO2������������ԭ��Ӧ�����ݵ����غ��֪��������ClO2�ͻ�ԭ����Cl�������ʵ���֮��Ϊ4��1������ԭ���غ㽫����ʽ��ƽ���ɣ���������ΪCl2��
�������ƶ��õ�����A(��H2SO4��Һ)��(4)���������֪��Ӧ��ΪNaClO2��HCl������NaClO2��ClԪ�صĻ��ϼ�Ϊ��3�ۣ��ȿ�����Ҳ�ɽ��ͣ��ʷ�ӦΪNaClO2������������ԭ��Ӧ�����ݵ����غ��֪��������ClO2�ͻ�ԭ����Cl�������ʵ���֮��Ϊ4��1������ԭ���غ㽫����ʽ��ƽ���ɣ���������ΪCl2��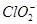 ��HCl�����˹��з�Ӧ�����ݵ����غ��֪��Ӧ��
��HCl�����˹��з�Ӧ�����ݵ����غ��֪��Ӧ��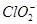 ��HCl�����ʵ���֮��Ϊ3��1������ԭ���غ㽫����ʽ��ƽ���ɣ�(5)���ʹ���ΪNaClO2������������ԭ��Ӧ�����۱��������Fe2����Ӧʱ��Ԫ�����ն�ת��ΪCl������ת�Ƶ�������ȡ�
��HCl�����ʵ���֮��Ϊ3��1������ԭ���غ㽫����ʽ��ƽ���ɣ�(5)���ʹ���ΪNaClO2������������ԭ��Ӧ�����۱��������Fe2����Ӧʱ��Ԫ�����ն�ת��ΪCl������ת�Ƶ�������ȡ�
��ϰ��ϵ�д�
�����Ŀ
 HBr��HBrO����Ҫʹ��ˮ����ɫ��dz���ɲ�ȡ�Ĵ�ʩ�Ǽ���NaI����
HBr��HBrO����Ҫʹ��ˮ����ɫ��dz���ɲ�ȡ�Ĵ�ʩ�Ǽ���NaI����

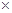 10-2mol��L-1��II�з�Ӧ����NaClO2��Һ(������NaOH)��pH=13������Һ��
10-2mol��L-1��II�з�Ӧ����NaClO2��Һ(������NaOH)��pH=13������Һ�� = ��
= ��