��Ŀ����
ijУ����С���ѧ���������ű���ʳ��ˮ�ķ����ռ���һƽ����ƿ��������ͬʱ�Ʊ���һƽ����ƿ�ı�����ˮ��̽�������������ʵ�顣�밴��Ҫ������������⣺
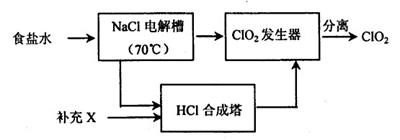
(1)����ͼ��װ��ʵ��װ�ã�U�ι���ʢ����ɫīˮ��A��B����Һ����ƽ��ƽ����ƿʢ��������ͨ����Һ©����ƽ����ƿ�еμ���������������Һ���۲쵽ʵ�������� �� �������ԭ�� ��
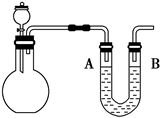
(2)��ͼ��ʾ��ƽ����ƿʢ��������ˮ�����չ����䵽ʢ�б�����ˮ��װ��ʱ���ɹ۲쵽ƽ����ƿ�������ݲ���������һ��ʱ�����Һ��ɫ��dz���������������ԭ���� ������ˮ�в��ٲ�������ʱ��ijѧ��������÷�Ӧ�����������壬��ͬѧ�ɲ�ȡ�ĺ��������� ��

(1)����ͼ��װ��ʵ��װ�ã�U�ι���ʢ����ɫīˮ��A��B����Һ����ƽ��ƽ����ƿʢ��������ͨ����Һ©����ƽ����ƿ�еμ���������������Һ���۲쵽ʵ�������� �� �������ԭ�� ��

(2)��ͼ��ʾ��ƽ����ƿʢ��������ˮ�����չ����䵽ʢ�б�����ˮ��װ��ʱ���ɹ۲쵽ƽ����ƿ�������ݲ���������һ��ʱ�����Һ��ɫ��dz���������������ԭ���� ������ˮ�в��ٲ�������ʱ��ijѧ��������÷�Ӧ�����������壬��ͬѧ�ɲ�ȡ�ĺ��������� ��

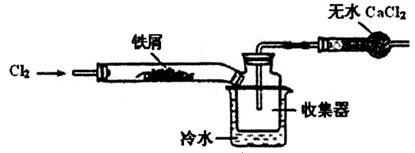
(1)ƽ����ƿ���������ɫ��dz��U�ι�A��ˮλ������B��ˮλ�½���Cl2������������Һ��Ӧ������������ɫ��dz��ͬʱƽ����ƿ������ѹǿ��С��ʹU�ι�A��ˮλ������B��ˮλ�½�
(2)��ˮ�к��д����ᣬ����������ֽ⣬���������������ʹƽ��Cl2��H2O HCl��HClO�����ƶ�����ˮ���������Ӽ��٣�������Һ��ɫ��dz����ƽ����ƿ���ϲ���Ƭ��ȡ���������������ϣ���ȥ����Ƭ���������ǵ�ľ������ƽ����ƿ��ƿ�ڣ�ľ����ȼ
HCl��HClO�����ƶ�����ˮ���������Ӽ��٣�������Һ��ɫ��dz����ƽ����ƿ���ϲ���Ƭ��ȡ���������������ϣ���ȥ����Ƭ���������ǵ�ľ������ƽ����ƿ��ƿ�ڣ�ľ����ȼ
(2)��ˮ�к��д����ᣬ����������ֽ⣬���������������ʹƽ��Cl2��H2O
 HCl��HClO�����ƶ�����ˮ���������Ӽ��٣�������Һ��ɫ��dz����ƽ����ƿ���ϲ���Ƭ��ȡ���������������ϣ���ȥ����Ƭ���������ǵ�ľ������ƽ����ƿ��ƿ�ڣ�ľ����ȼ
HCl��HClO�����ƶ�����ˮ���������Ӽ��٣�������Һ��ɫ��dz����ƽ����ƿ���ϲ���Ƭ��ȡ���������������ϣ���ȥ����Ƭ���������ǵ�ľ������ƽ����ƿ��ƿ�ڣ�ľ����ȼ(1)Cl2�ǻ���ɫ���壬Cl2����������������Һ��Ӧ�����IJ���Cl2��������ɫ��dz��ͬʱƽ����ƿ������ѹǿ��С��(2)���������ֽ⣬����HCl��������ƽ��Cl2��H2O??HCl��HClO���ƣ�������ˮ��Ӧ�ֻ���������ᣬ������ˮ���������Ӽ��١�ʹ�ô����ǵ�ľ������������

��ϰ��ϵ�д�
�����Ŀ

 NaClO3+3H2
NaClO3+3H2 ��
��