��Ŀ����
����Ŀ��CH4����һ����Ҫ����Դ��Ҳ��һ����Ҫ�Ļ���ԭ�ϡ�
��1��������·ֽ�����������̼�����ܱ������н��д˷�ӦʱҪͨ����������ʹ���ּ���ȼ�գ���Ŀ����________��
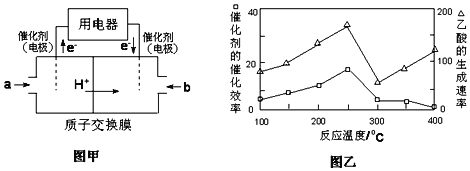
��2����CH4Ϊȼ�Ͽ���Ƴɽṹ������ת���ʸߡ��Ի�������Ⱦ��ȼ�ϵ�أ��乤��ԭ����ͼ����ʾ����ͨ��a����ĵ缫����Ϊ_____��ͨ��b����ĵ缫��ӦʽΪ____�������ӽ���Ĥֻ����H+ͨ����
��3����һ���¶Ⱥʹ��������£�CH4��CO2��ֱ��ת�������ᣬ����ʵ������������һ���о�����
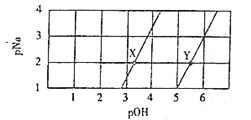
���ڲ�ͬ�¶��£������Ĵ�Ч�������������������ͼ����ʾ����÷�Ӧ������¶�Ӧ������__���ҡ�
�ڸ÷�Ӧ��������Ч�ɷ�Ϊƫ������ͭ��CuAlO2�����������CuAlO2�ܽ���ϡ���������������β��ų�NO���壬�����ӷ���ʽΪ___________��
��4��CH4��ԭ���Ǵ���NOx�����һ�ַ�������֪һ��������CH4��NOx���巴Ӧת��ΪN2��CO2������״����8.96LCH4�ɴ���22.4LNOx���壬��xֵΪ________��
���𰸡��ṩCH4�ֽ���������� ���� O2+4H++4e-=2H2O 250�� 3CuAlO2+16H++NO3-=3Cu2++3Al3++8H2O+NO�� 1.6
��������
��1������ֽ���Ҫ������ȼ�տ��ṩ����������
��2����ͼ��֪��ͨ������a��һ�˷���������Ӧ����Ӧͨ����飬�ü�Ϊ������ͨ��bΪ��������õ��ӣ���������������ˮ��
��3���ٸ������ᷴӦ��������������ѡ��
��CuAlO2�ܽ���ϡ���������������β��ų�NO���壬���ɵ���Ϊ������������ͭ����Ӧ����ˮ���ɣ���ƽ��д���ӷ���ʽ��
��4�����ݵ���ת���غ���㡣
(1)������·ֽ�����������̼�����ܱ������н��д˷�ӦʱҪͨ����������ʹ���ּ���ȼ�գ���Ŀ���ǣ��ṩCH4�ֽ�������������ʴ�Ϊ���ṩCH4�ֽ������������
(2)��ͼ��֪��ͨ������a��һ�˷���������Ӧ����Ӧͨ����飬�ü�Ϊ������ͨ��bΪ��������õ��ӣ���������������ˮ�������缫��ӦʽΪ��O2+4H++4e-=2H2O���ʴ�Ϊ��������O2+4H++4e-=2H2O��
(3)��250��ʱ���ᷴӦ����������ԣ���ѡ��250�棬�ʴ�Ϊ��250�棻
��CuAlO2�ܽ���ϡ���������������β��ų�NO���壬���ɵ���Ϊ������������ͭ����Ӧ����ˮ���ɣ���Ӧ���ӷ���ʽΪ��3CuAlO2+16H++NO3-=3Cu2++3Al3++8H2O+NO�����ʴ�Ϊ��3CuAlO2+16H++NO3-=3Cu2++3Al3++8H2O+NO����
(4)���ݵ���ת���غ㣬��8.96L��[4(4)]=22.4L��2x�����x=1.6���ʴ�Ϊ��1.6��

����Ŀ�����������ǿ�ѧѧϰ����Ҫ����֮һ������Ԫ�صĸ������ʿɹ�������Ϊ���±���ʾ�ı���(����)��
8O | 16S | 34Se | 52Te | |
�����۵�(��) | ��218.4 | 113 | 450 | |
���ʷе�(��) | ��183 | 444.6 | 685 | 989 |
��Ҫ���ϼ� | ��2 | ��2����4����6 | ��2����4����6 | |
ԭ�Ӱ뾶 | ������ | |||
������H2��Ӧ��� | ��ȼʱ���� | ���Ȼ��� | �����ѻ��� | ����ֱ�ӻ��� |
����ݱ��ش��������⣺
(1)�����۵㷶Χ������___________��
(2)�ڵĻ��ϼۿ�����__________��
(3)�������н�ǿ��________(������������������ԭ����)����˷��ڿ����г��ڱ����ױ��ʣ�����ܷ�����Ӧ�Ļ�ѧ����ʽΪ_______________��
(4)��ҵ��Al2Te3�������Ʊ�H2Te��������л�ѧ����ʽ��______Al2Te3��______=____Al(OH)3��______H2Te��