��Ŀ����
2��һ�������ºϳ���ϩ��6H2��g��+2CO2��g��$\stackrel{����}{?}$C2H4��g��+4H2O��g��
��1����ϩ�ǷǼ��Է��ӣ�����Ի�Ǽ��ԣ�
��2�����ڷ���ʽ�ϱ���÷�Ӧ����ת�Ƶķ������Ŀ��
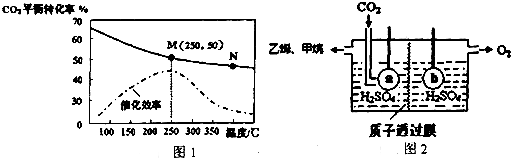
��3���¶ȶ�CO2��ƽ��ת���ʺʹ�����Ч�ʵ�Ӱ����ͼ1������˵����ȷ����B��
A���˷�Ӧ���¶����ߣ���Ӧ����һ���ӿ�
B��ƽ�ⳣ����KM��KN
C��M��CO2��ƽ��ת�������¶ȡ������Ĺ�ͬӰ��
��4����Ͷ�ϱ�n��H2����n��CO2��=3��1��ͼ1��M����ϩ���������Ϊ7.7% ��������λ��Ч���֣���
��5����ⷨ��ȡ��ϩװ����ͼ2���缫a�ӵ�Դ�ĸ�����������ϩ�ĵ缫��ӦʽΪ2CO2+12H++12e-��CH2=CH2+4H2O��
�������33.6L����״����CO2��Ӧʱ������������Һ������|��m��|-|��m��|=36g������ϩ�IJ���Ϊ66.7%
���� ��1��������������غ�Ϊ�Ǽ��Է��ӣ�����������IJ��غ�Ϊ���Է��ӣ���Ϸ��ӿռ乹���жϣ�
��2��������0�۱�Ϊ+1�ۣ�C��+4�۱�Ϊ-2�ۣ��ݷ�Ӧǰ�ϼ۱仯�����ת�Ƶķ������Ŀ��
��3��A���¶�����ѧ��Ӧ���ʼӿ죬�����Ĵ�Ч�ʽ��ͣ�
B���÷�Ӧ�Ƿ��ȷ�Ӧ������ƽ�������ƶ���
C������ֻӰ�컯ѧ��Ӧ���ʣ���Ӱ��ƽ���ƶ���
��4��Ͷ�ϱ�n��H2����n��CO2��=3��1��M��ʱ��������̼��ת����Ϊ50%����������ת����Ҳ��50%���ݴ˷�����
��5����ͼ�����������������¶�����̼���������ϩ���������������������ɣ�����������ϩ�����ݵ����غ���м��㼴�ɣ�
��� �⣺��1����ϩ������CԪ�ص�����������Ϊ4�����ϼ�Ϊ-4����������ȫ���ɼ����ռ�ṹΪƽ�������Σ�������ɵ������غϣ����ڷǼ��Է��ӣ��ʴ�Ϊ���Ǽ��ԣ�
��2��������0�۱�Ϊ+1�ۣ�C��+4�۱�Ϊ-2�ۣ�����6mol������Ӧת��12mol���ӣ� ��
��
�ʴ�Ϊ�� ��
��
��3��A����ѧ��Ӧ�������¶ȵ����߶��ӿ죬�����Ĵ�Ч�ʽ��ͣ����Է�Ӧ���ʿ��ܼ�С����A����
B������ƽ�������ƶ�����ѧƽ�ⳣ����С����B��ȷ��
C������ֻӰ�췴Ӧ���ʣ���Ӱ��ƽ���ƶ���ת���ʣ���C����
�ʴ�Ϊ��B��
��4���������������Ͷ�����̼�ֱ�Ϊ3mol��1mol����M��ʱ��������1.5mol��������̼0.5mol����ϩ0.25mol��ˮ����1mol������M��ʱ��ϩ���������Ϊ$\frac{0.25}{1.5+0.5+0.25+1}$��100%=7.7%���ʴ�Ϊ��7.7%��
��5����ͼ�����������������¶�����̼���������ϩ���������������������ɣ�����������ϩ������a�ӵ�Դ�ĸ�����������ԭ��Ӧ�������缫��ӦʽΪ2CO2+12H++12e-��CH2=CH2��4H2O���õ缫��������60g�������Ϸ����缫��Ӧ��12OH--12e-=3O2��+6H2O���õ缫������С96����������������Һ������|��m��|-|��m��|=36g����ת��12mol���ӣ����ݵ缫��Ӧʽ��2CO2+12H++12e-��CH2=CH2+4H2O��������ϩ�����ʵ�����1mol���������33.6L����״������1.5molCO2��Ӧʱ��������ϩ�����ʵ�����0.75mol��������ϩ�IJ�����75%���ʴ�Ϊ������2CO2+12H++12e-��CH2=CH2+4H2O��75%��
���� ���⿼���˸�˹���ɵ�Ӧ�á���ͼ�������ѧ��Ӧ���ʵ�Ӱ�����ء�ƽ����㡢ƽ���ƶ����缫��Ӧʽ��д����Ŀ�ѶȽϴ�

| A�� | 1mol/L�����ƣ�CH3COONa����Һ��CH3COO-��ĿС��NA | |
| B�� | �ڱ�״���£�11.2L��������ˮ���Ƴ�1L��Һ��NH4+������ĿΪ0.5NA | |
| C�� | �ڱ�״���£�������ΪNA��HF������ռ���Ϊ2.24L | |
| D�� | ��Zn+2HNO3+NH4NO2�TZn��NO3��2+N2��+3H2O��Ӧ����5.6g N2�����£���ת�Ƶ�����ΪNA |

| A�� | ����A������ | |
| B�� | ά����A����������Ʒ�����Ӧ�������� | |
| C�� | ά����A����ʹ����KMnO4��Һ��ɫ | |
| D�� | ά����A�ܷ���ȡ�����ӳɡ�������Ӧ |
 ������˵����ȷ���ǣ�������
������˵����ȷ���ǣ�������| A�� | 1mol���л���������3mol H2�����ӳɷ�Ӧ | |
| B�� | ��ʹ����KMnO4��Һ��ɫ | |
| C�� | 1mol���л���ֱ��������Ľ����ơ�̼��������Һ��Ӧ������1mol���� | |
| D�� | �����ụΪͬϵ�� |
 ��X��һ���������ܷ����ķ�Ӧ��acd��ѡ����ĸ����
��X��һ���������ܷ����ķ�Ӧ��acd��ѡ����ĸ����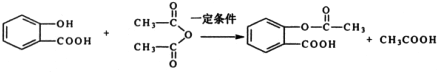

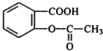
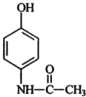 ����ŵ���Ľṹ��ʽ��
����ŵ���Ľṹ��ʽ��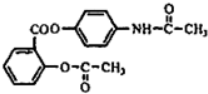
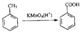 ���ڼ����ӿ��Ժϳ��л���X���ϳ�·����ͼ���ڼ����ӡ�W��X�Ľṹ����W�Ľṹ��ʽ��
���ڼ����ӿ��Ժϳ��л���X���ϳ�·����ͼ���ڼ����ӡ�W��X�Ľṹ����W�Ľṹ��ʽ��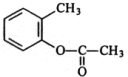 ��
��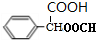 ��
��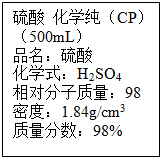 ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ�
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ�