��Ŀ����
10������ͭ�۾������������ʣ�����������������ij�о���ѧϰС����CuSO4•5H2OΪԭ���Ʊ�����ͭ�ۣ���������ͼ1����֪���ٷ�ɢ��Ϊ�л�������һ�˿�����ˮ�У���һ�˿����������ӱ����γ��л���Ĥ��ֹ�����žۣ�
��EDTA�����뷴Ӧ��������Ϊ��ֹCu2+�ڼ��������³�����
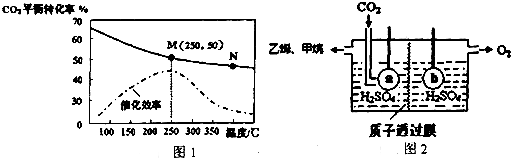
��1��Ϊ�˷�ֹ�Ʊ����������������žۣ��ڢٲ��м���ķ�ɢ��������bc��
a�����ȹ��飨SiHCl3��b�������� c�������� d����������
��2���ڢڲ���Ӧ�����ӷ���ʽ�ǡ�Cu2++��BH4-+��8OH-=��BO2-+��Cu+��H2O
��3���ڢ۲�ʵ�������Ҫ�IJ�������Ϊ©�����ձ�����������ȷ����Ʒ�ѳ�ָ���ķ����dz��������أ�
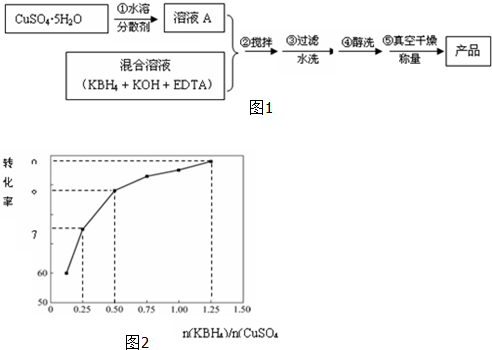
��4����֪����ͭת������KBH4��CuSO4���ʵ���֮�ȹ�ϵ��ͼ2��ʾ����С����125g CuSO4•5H2OΪԭ�ϣ����յõ���Ʒ30.72g��������Һ�к�KBH40.625mol��
���� ��1�����ȹ�����ˮ�⣬��������������ˮ��
��2��ͭ������BH4-�ڼ��������·�Ӧ����ͭ���ʺ�BO2-��
��3���ڢ۲�ʵ������ǹ��ˣ����γ���������ͬ��
��4��125gCuSO4•5H2O����ˮ�õ�A��A��KBH4��Ӧ�õ�ͭ���ʣ����衢���ˡ���ϴ����ո���õ���Ʒ������ͭԪ���غ㣬���ת���ʣ������ͼ�����KBH4�����ʵ�����
��� �⣺��1�����ȹ�����ˮ�⣬��������������ˮ����ɢ��Ϊ�л�������һ�˿�����ˮ�У���һ�˿����������ӱ����γ��л���Ĥ��ֹ�����ž����Ϊ�˷�ֹ�Ʊ����������������žۣ�����ķ�ɢ������������������������ʴ�Ϊ��bc��
��2��ͭ������BH4-�ڼ��������·�Ӧ����ͭ���ʺ�BO2-����Ӧ�����ӷ���ʽ��4Cu2++BH4-+8OH-=4Cu+BO2-+6H2O���ʴ�Ϊ��4Cu2++BH4-+8OH-=4Cu+BO2-+6H2O��
��3���ڢ۲�ʵ������ǹ��ˣ������Ҫ�IJ�������Ϊ©�������������ձ���ȷ����Ʒ�ѳ�ָ���ķ��������γ���������ͬ���ʴ�Ϊ��©���������������������أ�
��4��125gCuSO4•5H2OΪ0.5mol�����յõ�ͭ30.72gΪ$\frac{30.72g}{64g/mol}$=0.48mol����ת����Ϊ$\frac{0.48mol}{0.5mol}$=96%������ͼ�������KBH4Ϊ0.5��1.25mol���ʴ�Ϊ��0.625��
���� ���⿼���Ʊ�ʵ�鷽������ƣ��漰���ʵ��Ʊ������ӷ���ʽ����д�Լ����ʺ����ļ���ȣ���Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�
��1������D����������ƿ��
��2���ر�a��b����ͨ��ֱ�����ܵ�����ˮ����A����30���ӣ��Ʊ�1-�嶡�飮д���÷�Ӧ�Ļ�ѧ����ʽCH3CH2CH2CH2OH+NaBr+H2SO4 $\stackrel{����}{��}$ CH3CH2CH2CH2Br+NaHSO4+H2O��
��3�������ϣ�������Ӧ�������ﻹ�����У����ѡ�1-��ϩ���廯��ȣ�Ϩ��A���ƾ��ƣ�����ֱ�������Ϸ��������ӣ���a���������ȼ�����Ӧֱ����ȴ��ͨ��B��Cװ�ü��鲿�ָ����B��C��Ӧʢ�ŵ��Լ��ֱ��������������������Һ����ˮ��
��4����ʵ������У�����A��Һ������ɫ��ɺ�ɫ���ú�ɫ������Ũ���ᷴӦ�Ļ�ѧ����ʽΪC+2H2SO4��Ũ�� $\frac{\underline{\;\;��\;\;}}{\;}$CO2��+2SO2��+2H2O��������ֱ�����ܵ��϶�����һ����װ���ռ���ʯ�ҵĸ���ܣ�������Ⱦ������
��5������л�����������£�
| ���� | �۵�/0C | �е�/0C |
| 1-���� | -89.5 | 117.3 |
| 1-�嶡�� | -112.4 | 101.6 |
| ���� | -95.3 | 142.4 |
| 1-��ϩ | -185.3 | -6.5 |
��6����ʵ������ȡ1-������NaBr�ֱ�Ϊ7.4g��13.0g�������Ĵֲ��ᆳϴ�ӡ�������ٴ�����õ�9.6g 1-�嶡�飬��1-�嶡��IJ�����70%��
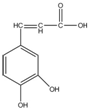
| A�� | ����ʽΪC9H5O4 | |
| B�� | ����ˮ���ܷ���ȡ����Ӧ�����ܷ����ӳɷ�Ӧ | |
| C�� | 1mol������������5mol���������ӳɷ�Ӧ | |
| D�� | ����Na2CO3��Һ��Ӧ����������NaHCO3��Һ��Ӧ |

| A�� | X��FeCl3��Һ�ܷ�����ɫ��Ӧ | |
| B�� | һ�������£�X�ܷ���ȡ����ˮ�⡢�������ӳɡ��Ӿۡ����۵ȷ�Ӧ | |
| C�� | 1mol X�ֱ���������NaOH��������Ӧ�����������5mol NaOH��7mol ���� | |
| D�� | �����X��Ӧʱ������Na��NaHCO3��Na2CO3����������ʵ���֮����3��1��1.5 |