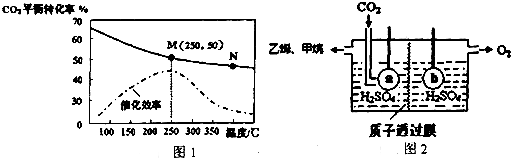
��Ŀ����
17����ͭ��������ͭ���������ͭ����Ҫ�ɷ�ΪCuFeS2��������Al2O3��SiO2������ͭ������ȡͭ���仯�������Ҫԭ��֮һ�������Ʊ������Ļ������1������ͭ����Ҫ���ջ�ͭ���䷴Ӧԭ��Ϊ2CuFeS2+O2$\frac{\underline{\;����\;}}{\;}$Cu2S+2FeS+SO2��CuFeS2��Cu��FeԪ�صĻ��ϼ۾�Ϊ+2�ۣ�����ԭ��Ԫ����Cu��O����Ԫ�ط��ţ�������Ӧ��ת��0.3mol����ʱ�����״���²���SO2�����Ϊ1.12L��
��2�����ջ�ͭ�������Cu2S�ɱ�ϡ��������ΪCu2+�����ʣ�HNO3�Ļ�ԭ����ΪNO����д��Cu2S��ϡ���ᷴӦ�����ӷ���ʽ3Cu2S+16H++4NO3-�T6Cu2++3S��+4NO��+8H2O���÷�Ӧ��ϡ�������������ԡ����ԣ�������ԡ�������ԭ�ԡ������ԡ�����
��3�����ջ�ͭ�ɵõ�Cu2O����21.6g Cu2O���뵽500mLijŨ�ȵ�ϡ�����У�����������ȫ��Ӧ������Cu��NO3��2��NO����������Һ�м���1.0mol•L-1��NaOH��Һ1.0L����ʱ��Һ�����ԣ�ԭ��������ʵ���Ũ��Ϊ2.2mol?L-1��
���� ��1��2CuFeS2+O2$\frac{\underline{\;����\;}}{\;}$Cu2S+2FeS+SO2�У�Cu��OԪ�صĻ��ϼ۽��ͣ�SԪ�صĻ��ϼ����ߣ�SԪ�صĻ��ϼ���-2������Ϊ+4�ۣ�
��2��Cu2S�ɱ�ϡ��������ΪCu2+�����ʣ�HNO3�Ļ�ԭ����ΪNO��Cu��SԪ�صĻ��ϼ����ߣ�NԪ�صĻ��ϼ۽��ͣ���������ͭ������������ԣ�����NO���������ǿ�����ԣ�
��3����ҺΪ���ԣ�������ΪNaNO3����Nԭ���غ��֪��NaOH��NaNO3����Ӧ����Һ��NO3-��n��Cu2O��=$\frac{21.6g}{144g/mol}$=0.15mol���ɵ����غ�ɼ������ɵ�NO��ԭ��Һ������Ϊn��NaOH��+n��NO����Ȼ����c=$\frac{n}{V}$���㣮
��� �⣺��1��2CuFeS2+O2$\frac{\underline{\;����\;}}{\;}$Cu2S+2FeS+SO2�У�Cu��OԪ�صĻ��ϼ۽��ͣ���ԭ��Ԫ����Cu��O��SԪ�صĻ��ϼ���-2������Ϊ+4�ۣ���֪����1mol����ת��6mol���ӣ���Ӧ��ת��0.3mol����ʱ�����״���²���SO2�����Ϊ0.3mol��$\frac{1}{6}$��22.4L/mol=1.12L���ʴ�Ϊ��Cu��O��1.12��
��2��Cu2S�ɱ�ϡ��������ΪCu2+�����ʣ�HNO3�Ļ�ԭ����ΪNO��Cu��SԪ�صĻ��ϼ����ߣ�NԪ�صĻ��ϼ۽��ͣ��ɵ��ӡ���ɼ�ԭ���غ��֪�����ӷ�ӦΪ3Cu2S+16H++4NO3-�T6Cu2++3S��+4NO��+8H2O����������ͭ������������ԣ�����NO���������ǿ�����ԣ����÷�Ӧ������������Ժ�ǿ�����ԣ�
�ʴ�Ϊ��3Cu2S+16H++4NO3-�T6Cu2++3S��+4NO��+8H2O�������ԡ����ԣ�
��3����ҺΪ���ԣ�������ΪNaNO3����Nԭ���غ��֪��NaOH��NaNO3����Ӧ����Һ��NO3-��������巴Ӧ����Һ�е�n��NO3-��=1L��1mol/L=1mol��
n��Cu2O��=$\frac{21.6g}{144g/mol}$=0.15mol���ɵ����غ��֪n��NO��=$\frac{0.15mol��2����2-1��}{��5-2��}$=0.1mol��ԭ��Һ������Ϊn��NaOH��+n��NO��=1mol+0.1mol=1.1mol��
ԭ��������ʵ���Ũ��Ϊ$\frac{1.1mol}{0.5L}$=2.2mol?L-1���ʴ�Ϊ��2.2mol?L-1��
���� ������ͭ���仯����Ϊ���忼��������ԭ��Ӧ�ļ��㣬Ϊ��Ƶ���㣬���շ����ķ�Ӧ����Ӧ��Ԫ�صĻ��ϼ۱仯���غ㷨Ӧ��Ϊ���Ĺؼ������ط����������������ۺϿ��飬��Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
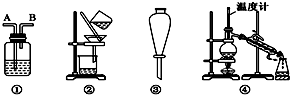
��1������D����������ƿ��
��2���ر�a��b����ͨ��ֱ�����ܵ�����ˮ����A����30���ӣ��Ʊ�1-�嶡�飮д���÷�Ӧ�Ļ�ѧ����ʽCH3CH2CH2CH2OH+NaBr+H2SO4 $\stackrel{����}{��}$ CH3CH2CH2CH2Br+NaHSO4+H2O��
��3�������ϣ�������Ӧ�������ﻹ�����У����ѡ�1-��ϩ���廯��ȣ�Ϩ��A���ƾ��ƣ�����ֱ�������Ϸ��������ӣ���a���������ȼ�����Ӧֱ����ȴ��ͨ��B��Cװ�ü��鲿�ָ����B��C��Ӧʢ�ŵ��Լ��ֱ��������������������Һ����ˮ��
��4����ʵ������У�����A��Һ������ɫ��ɺ�ɫ���ú�ɫ������Ũ���ᷴӦ�Ļ�ѧ����ʽΪC+2H2SO4��Ũ�� $\frac{\underline{\;\;��\;\;}}{\;}$CO2��+2SO2��+2H2O��������ֱ�����ܵ��϶�����һ����װ���ռ���ʯ�ҵĸ���ܣ�������Ⱦ������
��5������л�����������£�
| ���� | �۵�/0C | �е�/0C |
| 1-���� | -89.5 | 117.3 |
| 1-�嶡�� | -112.4 | 101.6 |
| ���� | -95.3 | 142.4 |
| 1-��ϩ | -185.3 | -6.5 |
��6����ʵ������ȡ1-������NaBr�ֱ�Ϊ7.4g��13.0g�������Ĵֲ��ᆳϴ�ӡ�������ٴ�����õ�9.6g 1-�嶡�飬��1-�嶡��IJ�����70%��
��Ӧ���ǴӺ��������ȡ�����Ҫ��Ӧ����Ӧ���Ǵ�������ʯ����ȡ�����Ҫ��Ӧ��
2NaI+MnO2+3H2SO4�T2NaHSO4+MnSO4+2H2O+I2��
2NaIO3+5NaHSO3�T2Na2SO4+3NaHSO4+H2O+I2��
�����й�˵����ȷ���ǣ�������
| A�� | NaI��NaIO3��һ���������ܷ�Ӧ����I2 | |
| B�� | I2�ڷ�Ӧ�����ǻ�ԭ����ڷ�Ӧ�������������� | |
| C�� | ������Ӧ�����ɵ�����I2ʱת�Ƶ�������� | |
| D�� | �����ԣ�MnO2��IO3-��SO42-��Mn42+ |
���Լ�����KMnO4/H+��NaOH��Һ���۱���Na2CO3��Һ��H2O����Na ��Br2/H2O����Br2/CCl4
��װ�ã�
| ѡ�� | ���� | �Լ� | װ�� |
| A | C2H6��C2H4�� | �� | �� |
| B | �������ӣ� | �� | �� |
| C | CH3COOC2H5��CH3COOH�� | �� | �� |
| D | �ױ������ױ��� | �� | �� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ����ͭƬ | B�� | ͨ������ | C�� | �μ�KSCN��Һ | D�� | �������� |
| A�� | ���ࡢ��֬�������ʶ�����C��H��OԪ�ع��ɵĸ߷��ӻ����� | |
| B�� | ���ۺ���ά�صķ���ʽ��ȫ��ͬ�����ǻ�Ϊͬ���칹�� | |
| C�� | ��֬����������һ�������¶����Է���ˮ�⣬��ˮ����ﲻͬ | |
| D�� | ú��Һ�����������������仯��������Ϊԭ���ѽ�ɵõ����������� |
 ��
�� �Ƚ�ϳɵģ����ij�������ͬʱ������4�ֻ��ţ�����̼ԭ�������٣�д������ܵĽṹ��ʽ�����ƣ�
�Ƚ�ϳɵģ����ij�������ͬʱ������4�ֻ��ţ�����̼ԭ�������٣�д������ܵĽṹ��ʽ�����ƣ� ������2��2��4-�������飮
������2��2��4-�������飮 ������2��3��3-�������飮
������2��3��3-�������飮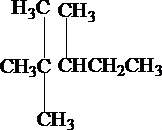 ������2��2��3-�������飮
������2��2��3-�������飮