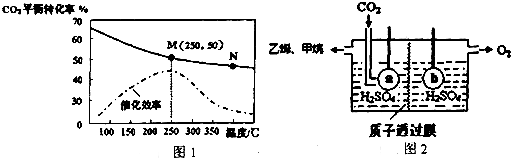
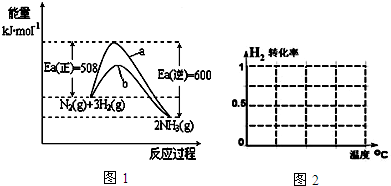
��Ŀ����
11��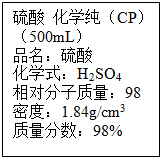 ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ�
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ������ø�Ũ��������100mL��1mol/L��ϡ���ᣮ�ɹ�ѡ�õ������У�
�ٽ�ͷ�ιܣ�����ƿ�����ձ�����ҩ�ף�����Ͳ����������ƽ��
��ش��������⣺
��1������ϡ����ʱ�����������в���Ҫʹ�õ��Тڢܢޣ�����ţ�����ȱ�ٵ������в�������100mL����ƿ��д�������ƣ���
��2����������100mL1mol/L��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ5.4mL������һλС��������ȡŨ����ʱӦѡ�â٣�ѡ���10mL����50mL����100mL��������Ͳ��
�������������ƫ�ߡ�����ƫ�͡�������Ӱ�족��
��1�����ݺӸǵ�תҡ�Ⱥ���Һ����ڿ̶��ߣ��ֵμ�����ˮ���̶ȣ���������ҺŨ�ȵ�Ӱ�죺ƫ�ͣ�
��2��������ƿ��ϴ����δ�����������ˮ���������Ƶ���ҺŨ����Ӱ�죻
��3������ʱ���۾����ӣ��������Ƶ���ҺŨ��ƫ�ͣ�
���� I����1����������һ�����ʵ���Ũ�ȵ���Һ���Ʋ���ѡ��ʹ����������ȱ�ٵ�������
��2������c=�����Ũ��������ʵ���Ũ�ȣ�����100mL1mol/L��ϡ��������������ʵ����������ҪŨ�������������ݼ�����ѡ����Ͳ�Ĺ��
II������c=$\frac{n}{V}$��ϲ������̶�n��V��Ӱ����������
��� �⣺I����1����100mL��1mol/L��ϡ��������Ʋ����У����㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡ���õ���ͷ�ιܣ������ձ���ϡ�ͣ����ò��������裬��ȴ��ת�Ƶ�100mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ��μ�����Һ������̶���ˮƽ���У��Ǻ�ƿ����ҡ�ȣ���Ҫʹ�õ�����Ϊ����Ͳ���ձ�����������100mL����ƿ����ͷ�ιܣ����Բ���Ҫ������Ϊ���ڢܢޣ���ȱ�ٵ�����Ϊ����������100mL����ƿ��
�ʴ�Ϊ���ڢܢޣ���������100mL����ƿ��
��2��ͼʾ��Ũ��������ʵ���Ũ��Ϊ��c=$\frac{1000��1.84��98%}{98}$mol/L=18.4mol/L������100mL1mol/L��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ��$\frac{1mol/L��0.1L}{18.4mol/L}$��0.0054L=5.4mL��Ӧ��ѡ�â�10mL��Ͳ��
�ʴ�Ϊ��5.4���٣�
II����1�����ݺӸǵ�תҡ�Ⱥ���Һ����ڿ̶����������ģ��ֵμ�����ˮ���̶ȣ��ᵼ����ҺŨ��ƫС���ʴ�Ϊ��ƫ�ͣ�
��2��������ƿ��ϴ����δ�����������ˮ���������Ƶ���ҺŨ����Ӱ�죬�ʴ�Ϊ����Ӱ�죻
��3������ʱ���۾����ӣ��������Ƶ���Һ�����ƫ����Ũ��ƫС���ʴ�Ϊ��ƫ�ͣ�
���� ���⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ������������е��Ѷȵ����⣬���������ǿ�������߿��������������У�ע������ԣ����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ������˼ά�������Ͻ��Ĺ淶ʵ�����������������ѵ�������������ע�������������ķ����뼼�ɣ�

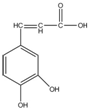
| A�� | ����ʽΪC9H5O4 | |
| B�� | ����ˮ���ܷ���ȡ����Ӧ�����ܷ����ӳɷ�Ӧ | |
| C�� | 1mol������������5mol���������ӳɷ�Ӧ | |
| D�� | ����Na2CO3��Һ��Ӧ����������NaHCO3��Һ��Ӧ |
| A�� | ����ͭƬ | B�� | ͨ������ | C�� | �μ�KSCN��Һ | D�� | �������� |
| A�� | ִ�С��������Ҫ��Ϊ�˽�Լ��Դ | |
| B�� | ���ʯ����Ȼ����Ӳ���������ʣ�������������������Ӧ | |
| C�� | ���ö����ЧӦ�����ֵ�������Һ����������Һ | |
| D�� | ��ҵ���������У��������̴ѿ��Խ��͵���SO2��Ũ�ȣ����ٿ�����Ⱦ |

| A�� | X��FeCl3��Һ�ܷ�����ɫ��Ӧ | |
| B�� | һ�������£�X�ܷ���ȡ����ˮ�⡢�������ӳɡ��Ӿۡ����۵ȷ�Ӧ | |
| C�� | 1mol X�ֱ���������NaOH��������Ӧ�����������5mol NaOH��7mol ���� | |
| D�� | �����X��Ӧʱ������Na��NaHCO3��Na2CO3����������ʵ���֮����3��1��1.5 |
| A�� | ����Ӧ����� | B�� | ����ѹǿ | C�� | �����¶� | D�� | ������������� |