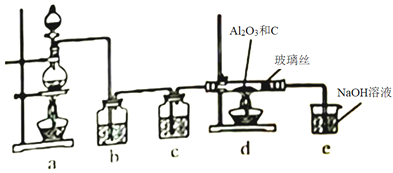
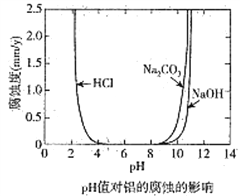
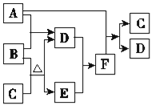
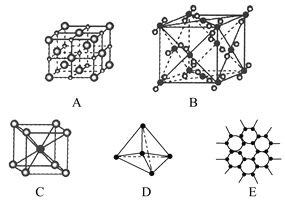
��Ŀ����
����Ŀ��������Ŀ��Ϣ������з���ʽ��
(1)��(Ti)��Ϊ�������������Խ��Խ�������ǵĹ�ע���ؿ��к�������ʯ֮һ�ǽ��ʯ(TiO2)��Ŀǰ���ģ�����ķ����ǣ�
��һ�������ʯ��̿�ۻ�ϣ��ڸ��������£�ͨ��Cl2�Ƶ�TiCl4��һ�ֿ�ȼ���壬�÷�Ӧ�Ļ�ѧ����ʽΪ��____________________________________________��
�ڶ�����������������У��ù�����þ�ڼ�����������TiCl4��Ӧ�Ƶý����ѡ�д���˷�Ӧ�Ļ�ѧ����ʽ��____________________________________________��
(2)��100mL 0.1mol/L��NaOH��Һ����224mLCO2����(��״��)��ǡ����ȫ���ա��ٽ�������Һ����100mL 0.1mol/L����������Һ�С���д��������Һ������������Һ��Ӧ�����ӷ���ʽ��_________________________________________________��
���𰸡� TiO2+2C+2Cl2 ![]() TiCl4+2CO 2Mg+TiCl4
TiCl4+2CO 2Mg+TiCl4![]() 2MgCl2+Ti Ca2++HCO3- + OH- = CaCO3��+ H2O
2MgCl2+Ti Ca2++HCO3- + OH- = CaCO3��+ H2O
��������
��1����һ��:����Ԫ���غ㼰ԭ���غ�ȷ����һ��������,�ٸ��ݷ�Ӧ������P��Ӧ������д����ʽ��
�ڶ���:���ݷ�Ӧ������P��Ӧ������д����ʽ��
��2����100mL0.1mol/L��NaOH����224mLCO2����(��״��)��ǡ����ȫ���ա�n(NaOH)=0.1mol/L��0.1L=0.01mol��n(CO2)=0.224L/22.4L/mol=0.01mol������ǡ�÷�Ӧ����NaHCO3���ٽ�������Һ����100mL0.1mol/L����������Һ��,�����������ʵ���0.1mol/L��0.1 L=0.01mol������̼����������������1:1��ȫ��Ӧ����̼��ơ��������ƺ�ˮ���ݴ�д���÷�Ӧ�����ӷ���ʽ��
(1)��һ��:�ڸ���ʱ,�����ʯTiO2��̿�ۻ�ϲ�ͨ�������Ƶ�TiCl4��һ�ֿ�ȼ����,����Ԫ���غ㼰ԭ���غ�֪,��������CO,���Է�Ӧ����ʽΪ��TiO2+2C+2Cl2 ![]() TiCl4+2CO��������������У��ù�����þ�ڼ�����������TiCl4��Ӧ�Ƶý����ѣ������û���Ӧ����Ӧ�Ļ�ѧ����ʽ��2Mg+TiCl4
TiCl4+2CO��������������У��ù�����þ�ڼ�����������TiCl4��Ӧ�Ƶý����ѣ������û���Ӧ����Ӧ�Ļ�ѧ����ʽ��2Mg+TiCl4![]() 2MgCl2+Ti ��
2MgCl2+Ti ��
����������������ǣ�TiO2+2C+2Cl2 ![]() TiCl4+2CO��2Mg+TiCl4
TiCl4+2CO��2Mg+TiCl4![]() 2MgCl2+Ti ��
2MgCl2+Ti ��
(2)��100mL0.1mol/L��NaOH����224mLCO2����(��״��)��ǡ����ȫ���ա�n(NaOH)=0.1mol/L��0.1L=0.01mol��n(CO2)=0.224L/22.4L/mol=0.01mol,����ǡ�÷�Ӧ����NaHCO3���ٽ�������Һ����100mL0.1mol/L����������Һ��,�����������ʵ���0.1mol/L��0.1 L=0.01mol������̼����������������1:1��ȫ��Ӧ����̼��ơ��������ƺ�ˮ����Ӧ�����ӷ���ʽΪ: Ca2++HCO3-+OH- =CaCO3��+H2O��
������������������Ca2++HCO3-+OH- = CaCO3��+H2O��

����Ŀ��X��Y��Z��W��Ԫ�����ڱ�ǰ�������е����ֳ���Ԫ�����������Ϣ���±���
Ԫ�� | �����Ϣ |
X | X�Ļ�̬ԭ�Ӻ���3���ܼ����е�������ÿ���ܼ��ϵĵ�������� |
Y | ���³�ѹ����Y�����ǵ���ɫ���������ڻ�ɽ�ڸ������� |
Z | Z��Yͬ������Z�ĵ縺�Դ���Y |
W | W��һ�ֺ��ص�������Ϊ63��������Ϊ34 |
��1��Yλ��Ԫ�����ڱ���________���ڵ�________����Y��Z������������Ӧ��ˮ��������Խ�ǿ����________(д��ѧʽ)��
��2��W�Ļ�̬ԭ�Ӻ�������Ų�ʽ��___________________________________��
��3��W2Y�ڿ�������������W2O�Ļ�ѧ����ʽ��________________________��