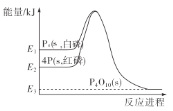
ЬтФПФкШн
ЁОЬтФПЁПдкШнЛ§ЮЊ2LЕФКуШнУмБеШнЦїжаЃЌЭЈШЫ0.02 mol O2(g)КЭ0.02 mol SO2(g)ЃЌдквЛЖЈЬѕМўЯТЗДгІЩњГЩSO3ЦјЬхЃЌЕБЗДгІНјааЕН5minЪБЃЌВтЕУШнЦїФкга0.012 mol SO3(g)ЁЃдђЃК
(1)5minЪБЃЌШнЦїФкn(O2 )=______c(SO2) =_______
(2)0~5 minФкЃЌвдO2БэЪОЕФЦНОљЗДгІЫйТЪv(O2)=______
(3)5minЪБЃЌШнЦїФкЦјЬхЕФзмЮяжЪЕФСПгыЗДгІЧАШнЦїФкЦјЬхЕФзмЮяжЪЕФСПжЎБШЮЊ___
(4)ШєЗДгІНјааЕН10minЪБДяЕНЦНКтЃЌДЫЪБc(SO2)+c(SO3) = _______
ЁОД№АИЁП0.014mol 0.004mol/L 0.0006molЁЄL-1ЁЄmin-1 17:20 0.01mol/L
ЁОНтЮіЁП
2LЕФКуШнУмБеШнЦїжаЃЌЭЈШЫ0.02 mol O2(g)КЭ0.02 mol SO2(g)ЃЌИљОнШ§ЖЮЪНЃК

МЦЫуn(O2 )ЁЂc(SO2) ЁЂv(O2)вдМА5minЪБЃЌШнЦїФкЦјЬхЕФзмЮяжЪЕФСПгыЗДгІЧАШнЦїФкЦјЬхЕФзмЮяжЪЕФСПжЎБШЁЃ
ЃЈ1ЃЉ2LЕФКуШнУмБеШнЦїжаЃЌЭЈШЫ0.02 mol O2(g)КЭ0.02 mol SO2(g)ЃЌИљОнЗДгІЕФШ§ЖЮЪНЃК

5minЪБЃЌШнЦїФкn(O2 )=0.014molЃЌ![]() ЃЌЙЪД№АИЮЊЃК0.014molЃЛ0.004mol/LЁЃ
ЃЌЙЪД№АИЮЊЃК0.014molЃЛ0.004mol/LЁЃ
ЃЈ2ЃЉ0~5minФкЃЌ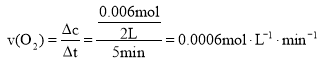 ЃЌЙЪД№АИЮЊЃК0.0006molЁЄL-1ЁЄmin-1ЁЃ
ЃЌЙЪД№АИЮЊЃК0.0006molЁЄL-1ЁЄmin-1ЁЃ
ЃЈ3ЃЉ5minЪБЃЌШнЦїФкЦјЬхЕФзмЮяжЪЕФСПгыЗДгІЧАШнЦїФкЦјЬхЕФзмЮяжЪЕФСПжЎБШЮЊЃК![]() ЃЌЙЪД№АИЮЊЃК17:20ЁЃ
ЃЌЙЪД№АИЮЊЃК17:20ЁЃ
ЃЈ4ЃЉИљОнжЪСПЪиКуЖЈТЩЃЌГфШы0.02mol SO2(g)ЃЌВПЗжЩњГЩSO3(g)ЃЌn(SO2)+n(SO3)=0.02molЃЌЬхЛ§ЮЊ2LЃЌдђc(SO2)+c(SO3)=0.01molL-1ЃЌЙЪД№АИЮЊЃК0.01molL-1ЁЃ

 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИЁОЬтФПЁПФГбЇЩњЮЊСЫЬНОПаПгыбЮЫсЗДгІЙ§ГЬжаЕФЫйТЪБфЛЏЃЌЫћдк100mLЯЁбЮЫсжаМгШызуСПЕФаПЗлЃЌгУХХЫЎМЏЦјЗЈЪеМЏЗДгІЗХГіЕФЧтЦјЃЌЪЕбщМЧТМШчЯТЃЈБэжаЦјЬхЬхЛ§ЮЊРлМЦжЕЃЌЧввбзЊЛЏЮЊБъзМзДПіЃЉЃК
ЪБМфЃЈminЃЉ | 1 | 2 | 3 | 4 | 5 |
ЧтЦјЬхЛ§ЃЈmLЃЉ | 50 | 120 | 232 | 290 | 310 |
ЂйФФвЛЪБМфЖЮЃЈжИ0ЁЋ1ЁЂ1ЁЋ2ЁЂ2ЁЋ3ЁЂ3ЁЋ4ЁЂ4ЁЋ5minЃЉЗДгІЫйТЪзюДѓ_____minЃЌдвђЪЧ_________________ЃЎ
ЂкФФвЛЖЮЪБЖЮЕФЗДгІЫйТЪзюаЁ_____minЃЌдвђЪЧ_____________ЃЎ
ЂлЧѓ2ЁЋ3ЗжжгЪБМфЖЮвдбЮЫсЕФХЈЖШБфЛЏРДБэЪОЕФИУЗДгІЫйТЪ_____________ЃЈЩшШмвКЬхЛ§ВЛБфЃЉ
ЂмШчЙћЗДгІЬЋМЄСвЃЌЮЊСЫМѕЛКЗДгІЫйТЪЖјгжВЛМѕЩйВњЩњЧтЦјЕФСПЃЌЫћдкбЮЫсжаЗжБ№МгШыЕШЬхЛ§ЕФЯТСаШмвКЃК
A.еєСѓЫЎЃЛB.NaClШмвКЃЛC.NaNO3ШмвКЃЛD.CuSO4ШмвКЃЛE.Na2CO3ШмвКЃЌФуШЯЮЊПЩааЕФЪЧ_____ЃЎ
ЁОЬтФПЁПЯТБэЪЧГЃЮТЯТМИжжГЃМћШѕЫсЕФЕчРыЦНКтГЃЪ§:
ШѕЫс | ЕчРыЗНГЬЪН | ЕчРыЦНКтГЃЪ§K |
CH3COOH | CH3COOH | K=1.6ЁС10-5 |
H2C2O4 | H2C2O4 HC2O4- | K1=5.9ЁС10-2 K2=6.4ЁС10-5 |
H2CO3 | H2CO3 | K1=4.4ЁС10-7 K2=5.6ЁС10-11 |
H2S | H2S | K1ЃН9.1ЁС10Ѓ8K2ЃН1.1ЁС10Ѓ15 |
ЛиД№ЯТСаЮЪЬт:
(1)ФГЮТЖШЯТЃЌДПЫЎжаЕФcЃЈH+ЃЉ=2ЃЎ0ЁС10-7mol/LЃЌдђДЫЪБШмвКжаcЃЈOH-ЃЉЮЊ_______mol/LЃЛДЫЪБЮТЖШ__________25 ЁцЃЈЬюЁАДѓгкЁБЃЌЁАаЁгкЁБЛђЁАЕШгкЁБЃЉЃЌШєЮТЖШВЛБфЃЌЕЮШыЯЁСђЫсЪЙcЃЈH+ЃЉ=5ЃЎ0ЁС10-6mol/LЃЌдђгЩЫЎЕчРыГіЕФcЃЈH+ЃЉЮЊ______mol/LЁЃ
(2)ЯТСаЫФжжРызгНсКЯH+ФмСІзюЧПЕФЪЧ______ЁЃ
AЃЎHCO3- BЃЎ C2O42- CЃЎ S2Ѓ DЃЎCH3COO-
(3)ИУЮТЖШЯТ1.0 molЁЄL-1ЕФCH3COOHШмвКжаЕФc(H+)=_____span> molЁЄL-1 ЁЁ
(4)ГЃЮТЯТЃЌМгЫЎЯЁЪЭ0.1 molЁЄL-1ЕФH2C2O4ШмвКЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ______ЃЉ
AЃЎШмвКжаn(HЃЋ)ЁСn(OHЃ)БЃГжВЛБф
BЃЎШмвКжаЫЎЕчРыЕФc(HЃЋ)ЁСc(OHЃ)БЃГжВЛБф
CЃЎШмвКжаc(HC2O4-)/c(H2C2O4)БЃГжВЛБф
DЃЎШмвКжаc(OHЃ)діДѓ
(5)НЋCH3COOHШмвКМгШыЩйСПNa2CO3ШмвКжаЃЌЗДгІЕФРызгЗНГЬЪНЮЊ__________________________ЁЃ