��Ŀ����
����Ŀ������������⣺
��1���������������ڵ���ʵ���_________�����ڷǵ���ʵ���_________���Ȳ��ǵ����Ҳ���Ƿǵ���ʵ���________________��
��ϡ���� ������ ��NaCl���� �����ᱵ ��CCl4����ͭ
��NaHCO3����CO2����HCl �ⰱ��
��2��д�����з�Ӧ�����ӷ���ʽ
��NaCl+AgNO3===AgCl��+ NaNO3�� _____________________________________��
��HCl+CH3COONa=== CH3COOH+NaCl��_____________________________________��
��Ba(OH)2��H2SO4��ϡ��Һ��� ��_____________________________________��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��д������CO![]() ��2H��===CO2����H2O��
��2H��===CO2����H2O��
��_____________________________________��
��_____________________________________��
���𰸡� �ۢܢߢ����� �ݢ�� �٢ڢ� Cl- + Ag+ = AgCl�� H+ + CH3COO�� = CH3COOH Ba2+ + 2OH- + 2H+ + SO42- = BaSO4�� + 2H2O Na2CO3 + 2HCl=2NaCl + H2O+ CO2�� K2CO3 + H2SO4 =K2SO4 +H2O+ CO2��
����������1������ˮ������״̬���ܹ�����Ļ������ǵ���ʣ�������ڵ���ʵ����Ȼ��ƹ��塢���ᱵ��̼�����ƺ��Ȼ��⣬��ѡ�ۢܢߢ�������ˮ������״̬�¶����ܹ�����Ļ������Ƿǵ���ʣ�������ڷǵ���ʵ������Ȼ�̼��������̼�Ͱ�������ѡ�ݢ�⣻�Ȳ��ǵ����Ҳ���Ƿǵ���ʵ���ϡ���ᡢ�����ͭ����ѡ�٢ڢ���
��2����NaCl+AgNO3===AgCl��+ NaNO3�����ӷ���ʽΪCl- + Ag+ =AgCl������HCl+CH3COONa===CH3COOH+NaCl�����ӷ���ʽΪH++CH3COO��=CH3COOH����Ba(OH)2��H2SO4��ϡ��Һ����������ᱵ��ˮ�����ӷ���ʽΪBa2+ +2OH- +2H+ +SO42-=BaSO4�� +2H2O��
��3�����ݷ���ʽ��֪����������Ӧ���ǿ�����̼���κ�ǿ�ᷴӦ���ɿ������Ρ�ˮ�Ͷ�����̼����˿�����Na2CO3 +2HCl=2NaCl +H2O+CO2����K2CO3 +H2SO4 =K2SO4 +H2O+CO2����

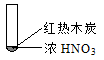
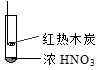
����Ŀ������ʵ���о��к���ɫ����������Աȷ������ý��۲���ȷ����
|
|
|
�� | �� | �� |
A�������еĺ���ɫ���壬�ƶϲ���������һ���ǻ������
B������ɫ���岻�ܱ�������ľ̿��Ũ��������˷�Ӧ
C������˵��Ũ������лӷ��ԣ����ɵĺ���ɫ����Ϊ��ԭ����
D��������������м���CO2���ɴ�˵��ľ̿һ����Ũ���ᷢ���˷�Ӧ