��Ŀ����
12��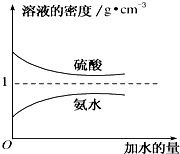 ��֪���ᡢ��ˮ���ܶ�������ˮ���Ĺ�ϵ��ͼ��ʾ�����������백ˮ��һ�ݣ�����ݱ�����Ϣ���ش��������⣺
��֪���ᡢ��ˮ���ܶ�������ˮ���Ĺ�ϵ��ͼ��ʾ�����������백ˮ��һ�ݣ�����ݱ�����Ϣ���ش��������⣺| ���ʵ����ʵ���Ũ��/mol•L-1 | ��Һ���ܶ�/g•cm-3 | |
| ���� | c1 | ��1 |
| ��ˮ | c2 | ��2 |
��2����������Ϊw1��������ˮ�������ϣ�������Һ�������������� $\frac{{w}_{1}}{2}$������ڡ���С�ڡ����ڡ�����ͬ����
��3�����ʵ���Ũ��Ϊc2 mol•L-1�İ�ˮ��$\frac{1}{5}$c2 mol•L-1�İ�ˮ��������ϣ�������Һ���ܶȴ��ڦ�2 g•cm-3��������Һ�����ʵ���Ũ�ȴ���$\frac{3}{5}$c2 mol•L-1����Ϻ���Һ������仯���Բ��ƣ���
��4����������NaOH��Ʒ1g����Ʒ������Na2CO3��ˮ��������50mL 1mol/L�������У���ַ�Ӧ����Һ�����ԣ��кͶ����������ȥ40mL 1mol/L��NaOH��Һ�������кͺ����Һ�����յõ�����g���壿
���� ��1����������һ�����ʵ���Ũ�ȵ���Һ�IJ���ѡ��������
��2������ϡ�Ͷ��ɣ�ϡ��ǰ��������������ʵ������������䣬�ݴ˼���ϡ�ͺ���Һ������������
��3��c2mol•L-1�İ�ˮ��$\frac{1}{5}$c2mol•L-1�İ�ˮ��������ϣ���Ϻ���Һ��Ũ��С��c2mol•L-1�İ�ˮ����ͼ��֪����ˮ��Ũ��Խ���ܶ�ԽС���ݴ��жϻ�Ϻ���Һ���ܶ����2g•cm-3��ϵ��
���ʵ���Ũ�ȷֱ�Ϊc2mol•L-1��$\frac{1}{5}$c2mol•L-1�İ�ˮ��������ϣ���c2mol•L-1��$\frac{1}{5}$c2mol•L-1�İ�ˮ������ֱ�ΪaL��bL����Ϻ���Һ�����Ϊ��a+b��L����ʾ����Ϻ�ˮ�����ʵ���Ũ�ȣ���ˮ��Ũ��Խ���ܶ�ԽС������V=$\frac{m}{��}$��֪a��b���ݴ��жϣ�
��4�����յõ��Ĺ���Ϊ�����ƣ�������������ʵ�����֪�����Ƶ����ʵ������ٸ���m=nM����������Ƶ��������ɣ�
��� �⣺��1������480mL����Һ����Ҫѡ��500mL������ƿ���������Ƹ�������Һ�õ���ʵ�������У��ձ�����������500 mL����ƿ����ͷ�ιܡ���Ͳ��
�ʴ�Ϊ��500 mL����ƿ����ͷ�ιܡ���Ͳ��
��2����������Ϊw1��������ˮ�������ϣ�ˮ������С��������Һ����������������С��ԭ������2������ϡ�ͺ������������������ $\frac{{w}_{1}}{2}$��
�ʴ�Ϊ�����ڣ�
��3��c2mol•L-1�İ�ˮ��$\frac{1}{5}$c2mol•L-1�İ�ˮ��������ϣ���Ϻ���Һ��Ũ��С��c2mol•L-1�İ�ˮ����ͼ��֪����ˮ��Ũ��Խ���ܶ�ԽС���ʻ�Ϻ���Һ���ܶȴ��ڦ�2g•cm-3��
���ʵ���Ũ�ȷֱ�Ϊc2mol•L-1��$\frac{1}{5}$c2mol•L-1�İ�ˮ��������ϣ���c2mol•L-1��$\frac{1}{5}$c2mol•L-1�İ�ˮ������ֱ�ΪaL��bL����Ϻ���Һ�����Ϊ��a+b��L����Ϻ�ˮ�����ʵ���Ũ��Ϊ��$\frac{a{c}_{2}+b��\frac{1}{5}{c}_{2}}{a+b}$=c2+$\frac{\frac{1}{5}b{c}_{2}-b{c}_{2}}{a+b}$=c2-$\frac{\frac{4}{4}{c}_{2}}{1+\frac{a}{b}}$����ˮ��Ũ��Խ���ܶ�ԽС������V=$\frac{m}{��}$��֪a��b����$\frac{\frac{4}{4}{c}_{2}}{1+\frac{a}{b}}$��$\frac{2}{5}$c2����c2-$\frac{\frac{4}{4}{c}_{2}}{1+\frac{a}{b}}$��$\frac{3}{5}$c2��
�ʴ�Ϊ�����ڣ����ڣ�
��4����������NaOH��Ʒ1g����Ʒ������Na2CO3��ˮ��������50mL 1mol/L�������У���ַ�Ӧ����Һ�����ԣ��кͶ����������ȥ40mL 1mol/L��NaOH��Һ���������ɵõ��Ĺ���Ϊ�����ƣ���������������غ��֪�����Ƶ����ʵ���Ϊ��1mol/L��0.05L=0.05mol���������յõ��������Ƶ�����Ϊ��142g/mol��0.05mol=7.1g��
�����յõ��Ĺ��������Ϊ7.1 g��
���� ���⿼�������ʵ���Ũ�ȡ�����������������ѧ����ʽ�ļ��㣬��Ŀ�Ѷ��еȣ�����֪ʶ��϶ࡢ�������ϴ�ֿ���ѧ���ķ�����������ѧ����������

 ���ݼ���ϵ�д�
���ݼ���ϵ�д�| A�� | ���� | B�� | ��ͷ | C�� | ������ | D�� | ��Ȫˮ |
����ʹ����KMnO4��Һ��ɫ��
�ڿɷ����ӳɷ�Ӧ��
�ۿ�����ˮ��
�ܿ����ڱ��У�
������Ũ������ŨH2SO4�����·���ȡ����Ӧ��
�����е�ԭ�ӿ��ܹ�ƽ�森
| A�� | �٢ڢۢܢ� | B�� | �٢ڢݢ� | C�� | �٢ڢܢݢ� | D�� | ȫ�� |
| A�� | 85g | B�� | 79g | C�� | 116g | D�� | 58g |
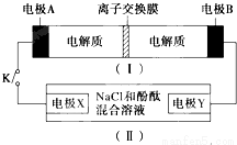
| A�� | �պ�Kʱ��X�ĵ缫��ӦʽΪ��2H++2e-�TH2�� | |
| B�� | �պ�Kʱ��A�缫��ӦʽΪ��NiO2+2e-+2H+�TNi��OH��2 | |
| C�� | ��װ�ã����ʱ��B�����뷴Ӧ�����ʱ����� | |
| D�� | ��װ�ã����ʱ��OH-ͨ�������ӽ���Ĥ������A�缫 |
| A�� | ��Һ��һ������Al3+��Ag+ | B�� | ��Һ��һ������Fe2+��Ag+ | ||
| C�� | ������һ������Fe | D�� | ������һ������Ag |
| A�� | 3molH2 | B�� | 98gH2SO4 | C�� | �����11.2LC2H6 | D�� | 3.01��1023��HCl |
