��Ŀ����
����Ŀ���±�Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�ء�

��ش��������⣺
��1��Ԫ�����Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ_________________��
��2����10��Ԫ�أ����е縺��������________(��Ԫ�ط���)��
��3��Ԫ�آڵ�һ���⻯��(�����к���6��ԭ��)����Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־���йظ��⻯����ӵ�˵����ȷ����________��
A.6��ԭ�Ӳ���ͬһƽ���� B.���ں��м��Լ��ķǼ��Է���
C.ֻ����4��sp2-s�Ħļ���1��P-P�Ħм� D.���⻯�������Cԭ�Ӳ���sp2�ӻ�
��4����ͼ��ʾΪѪ�쵰�ͼ��쵰�Ļ��Բ���(Ѫ����)�Ľṹʽ���˽ṹƬ���к��еĻ�ѧ����________(�����)��

A.���Ӽ� B.������ C.���Լ� D.�Ǽ��Լ� E.��λ�� G. �ļ� H.أ��
��5���±�Ϊԭ��������������Ķ�����Ԫ��A~F�ĵ�һ��������������ݡ�

��ش𣺱��е�D��E��F������������Ԫ��__________(��Ԫ�ط���)����A��B��CΪԭ���������������ͬ��������Ԫ�أ�������ʾB��A��C�ĵ�һ�����ܶ��Դ���ԭ����__________��
���𰸡�3d84s2 O BD CDEGH Na��Mg��Al B��np����ϵĵ��Ӱ�����������A��C�ͣ��������ȶ������Ե�һ�����ܱ�A��C��
��������
��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪC����ΪO����ΪMg����ΪAl����ΪS����ΪCl����ΪCa����ΪNi����ΪCu��
��1��Ԫ�آ�ΪNi��Ϊ28��Ԫ�أ���̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��
��2��Ԫ�طǽ�����Խǿ���縺��Խ����10��Ԫ���зǽ�������ǿ��ΪOԪ�أ���縺��������O��
��3��Ԫ�آ�ΪC������һ���⻯��(�����к���6��ԭ��) ����Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ� ����Ϊ����ʯ�ͻ�����չˮƽ�ı�־����Ϊ��ϩ�� A.��ϩ������6��ԭ����ͬһƽ������ѡ��A����B.���ں��м��Լ��ķǼ��Է��ӣ�ѡ��B��ȷ��C.����4��sp2-s�Ħļ���1��sp2- sp2�Ħļ���1��P-P�Ħм���ѡ��C����D.���⻯�������Cԭ�Ӳ���sp2�ӻ���ѡ��D��ȷ����ѡBD��
��4�����ݽṹʽ����Ԫ�غ�NԪ��֮�������λ����N��C֮�䣬C��H֮��ȴ��ڼ��Լ���C��C֮����ڷǼ��Լ������ڦҼ���C��C֮�����˫�����������Цм�����CDEGH��ȷ��
��5��ԭ��Խ����ʧ���ӣ����������ԽС������Ԫ�صĵ�һ�����ܱȽ�С����һ����������ʧȥ������A��B��C��һ�����ܽϴ�Ӧ���Ƿǽ���Ԫ�أ�D��E��F��һ�����ܽ�С�����DEFӦ���ǽ���Ԫ�أ����е�D��E��F������Na��Mg��Al����Ԫ�أ�ͬ���ڴ������ҵ�һ����������IIA>IIIA��VA>VIA��B��np����ϵĵ��Ӱ�����������A��C�ͣ��������ȶ������Ե�һ�����ܱ�A��C ��

 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�����Ŀ��H2S�ڽ������ӵļ���������ú��������������ҪӦ�á���ش�
��.��ҵ��һ���Ʊ�H2S�ķ������ڴ��������������£�����Ȼ����SO2��Ӧ��ͬʱ���������ܲ������ѭ���������
(1)�÷�Ӧ�Ļ�ѧ����ʽΪ_____________��
��.H2S�����ڼ��ͳ������������ӡ�
(2)H2S�ĵ�һ�����뷽��ʽΪ________��
(3)��֪��25 ��ʱ��Ksp(SnS)��1.0��10��25��Ksp(CdS)��8.0��10��27�����¶��£���Ũ�Ⱦ�Ϊ0.1 mol��L��1��CdCl2��SnCl2�Ļ����Һ��ͨ��H2S����Sn2����ʼ����ʱ����Һ��c(Cd2��)��________(��Һ����仯���Բ���)��
��.H2S��ú����ԭ����������̵���Ҫ�м��塣��Ӧԭ��Ϊ
��.COS(g)��H2(g) ![]() H2S(g)��CO(g)����H����7 kJ��mol��1��
H2S(g)��CO(g)����H����7 kJ��mol��1��
��.CO(g)��H2O(g) ![]() CO2(g)��H2(g)����H����42 kJ��mol��1��
CO2(g)��H2(g)����H����42 kJ��mol��1��
(4)��֪������1 mol�����еĻ�ѧ���������յ����������ʾ��
���� | COS(g) | H2(g) | CO(g) | H2S(g) | H2O(g) | CO2(g) |
����/(kJ��mol��1) | 1 319 | 442 | x | 678 | 930 | 1 606 |
����x��________��
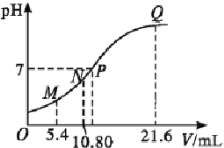
(5)��10 L�ݻ�������ܱ������г���1 mol COS(g)��1 mol H2(g)��1 mol H2O(g)����������������Ӧ��������������ʱ����ϵ��CO��ƽ������������¶�(T)�Ĺ�ϵ��ͼ��ʾ��
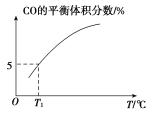
�������¶����ߣ�CO��ƽ���������_____(����������������С��)��ԭ��Ϊ_______
��T1��ʱ�����ƽ��ʱ��ϵ��COS�����ʵ���Ϊ0.80 mol������¶��£�COS��ƽ��ת����Ϊ_____����Ӧ����ƽ�ⳣ��Ϊ_____(������λ��Ч����)��
����Ŀ���±�ΪԪ�����ڱ���һ���֣���Ԫ�ط��Ż�ѧʽ�ش��������⡣
���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� | ||||
4 | �� | �� |
��1��д�������Ԫ����ɵ���ķ��ӵĵ���ʽ��________��
��2���ٵ���̬�⻯����������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽΪ_____�����������г���Ԫ���������Ӱ뾶��С����______�������ӷ��ţ���
��3���ڢۢݼ���Ԫ������������Ӧ��ˮ���������ǿ����_______ (�ѧʽ)��Ԫ�آߵļ��⻯��ĽṹʽΪ___________�����⻯���Ԫ�آܵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ_______________________��
��4�������������ͬ�����Ԫ�صĵ��ʢߵĵ�����ȼ�����ɵĻ�����ĵ���ʽ________���ܢ��Ԫ�ص����ӵĻ�ԭ����ǿ����˳��Ϊ____________�������ӷ��ţ���
����Ŀ���±���Ԫ�����ڱ����һ���֣����������Ԫ���Լ���Ӧ�������ش��й����⣺
���� ���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA |
1 | �� | ||||||
2 | �� | �� | �� | �� | |||
3 | �� | �� | �� | �� | |||
4 | �� |
��1��д���ɢٺ͢�����Ԫ����ɵIJ�������������ʵĻ�ѧʽ��________��_______��
��2����~���Ԫ��������������Ӧ��ˮ����������ǿ����Ļ�ѧʽΪ��_______��
��3���������������Ӧ��ˮ������������������ڼ��ȵ������������κ�ˮ�Ļ�ѧ����ʽΪ��___________________________________________________________��
��4�����ݵĵ���ͨ��ܵij����⻯���У���Ӧ���ң���Ӧ�Ļ�ѧ����ʽΪ��________��
��5����ͬ�����¢ݢޢߢ�����Ԫ�ص�ԭ�Ӱ뾶�ɴ�С��˳���ǣ�________________��
����Ŀ�������ҹ����гɹ����漰�����У���Ҫ�ɷ�Ϊͬ����Ԫ���γɵ����ǽ������ϵ���
|
|
|
|
A��4.03�״�ھ�̼���跴�侵 | B��2022�궬�»�۰����ٻ��� | C�������ε�Ų���̼������������ | D�������ö������ѺϽ�ɸ���� |
A. AB. BC. CD. D