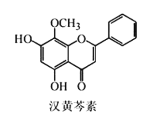
��Ŀ����
����Ŀ��������Һ�и�����Ũ�ȹ�ϵ��ȷ����
A.0.1 mol��L��1NaHSO4��Һ�У�c(Na��)��c(SO42-)��c(H��) ��c(OH��)
B.0.1 mol��L��1Na2S��Һ�У�2 c(Na��)��c(S2��)��c(HS��)��c(H2S)
C.0.1 mol��L��1NaHCO3��Һ�У�c(Na��)��c(H��)��c(HCO3-)��2c(CO32-)��c(OH��)
D.������������ʵ���Ũ�ȵ�������Һ������������Һ��Ϻ�C(Na��)��c(CH3COO��)��c(H��)��c(OH��)
���𰸡�C
��������
A��NaHSO4����ǿ�����ʽ�Σ����������ԣ�0.1 mol��L��1NaHSO4��Һ�У�c(Na��)��c(SO42-)��c(H��) ��c(OH��)����A����
B��Na2S����ǿ�������Σ�ˮ���Լ��ԣ����������غ��֪��0.1 mol��L��1Na2S��Һ�У�![]() c(Na��)��c(S2��)��c(HS��)��c(H2S)����B����
c(Na��)��c(S2��)��c(HS��)��c(H2S)����B����
C��NaHCO3��Һ�д��ڵ����ˮ���������̣����ݵ���غ��֪��0.1 mol��L��1NaHCO3��Һ�У�c(Na��)��c(H��)��c(HCO3-)��2c(CO32-)��c(OH��)����C��ȷ��
D��������������ʵ���Ũ�ȵ�������Һ������������Һ��Ϻ����ɴ�������Һ����������ӻ�ˮ�⣬��Һ�Լ��ԣ��������¹�ϵ��C(Na��)��c(CH3COO��)��c(OH��) ��c(H��)����D����
�ʴ�ѡC��

 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�����Ŀ����1����֪��2L���ܱ������н������¿��淴Ӧ�������ʵ��й��������£�
aA(g) |
| bB(g) |
| 2C(g) | |
��ʼ���ʵ���Ũ��/(mol��L-1)�� | 1.5 | 1 | 0 | ||
2sĩ���ʵ���Ũ��/(mol��L-1)�� | 0.9 | 0.8 | 0.4 |
��ش��������⡣
�ٸÿ��淴Ӧ�Ļ�ѧ����ʽ�ɱ�ʾΪ__��
��������B����ʾ0��2s��ƽ����Ӧ����Ϊ__��
�۴ӷ�Ӧ��ʼ��2sĩ��A��ת����Ϊ__��
��������ʵ�ܹ�˵��������Ӧ�ڸ��������Ѿ��ﵽ��ѧƽ��״̬����__������ţ�
A.vB�����ģ�=vC�����ɣ�
B.�������������ѹǿ���ֲ���
C.������������ܶȲ���
D.vA��vB��vC=3��1��2
E.����������C�����ʵ����������ֲ���
��2����п�����������Ǧ���أ����Ĺ��ɲ�����п��������ij�ֵ������Һ���������ܷ�Ӧ����ʽ��2Zn+O2=2ZnO����õ�صĸ���������__��
�����ASES��˾��Ƶ�����������DZͧ��Һ��-Һ��ȼ�ϵ�ص�ʾ��ͼ��ͼ����ȼ�ϵ�ع���ʱ����ص��ܷ�Ӧ����ʽΪ__�������ĵ缫��ӦʽΪ__��

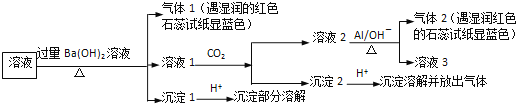
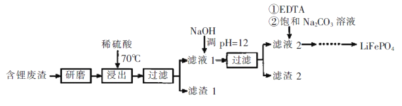
����Ŀ���ú�﮷�������Ҫ����Ԫ�صĺ�����Li 3.50% Ni 6.55% Ca 6.41% Mg 13.24%)�Ʊ�Li2CO3,�������Ʊ�﮵�ص���������LiFePO4�����ֹ����������£�
���ϣ�i��Һ1����Һ2�в�������Ũ�ȣ�g��L��1��
Li�� | Ni2�� | Ca2�� | Mg2�� | |
��Һ1 | 22.72 | 20.68 | 0.36 | 60.18 |
��Һ2 | 21.94 | 7.7��10��3 | 0.08 | 0.78��10��3 |
ii��EDTA�ܺ�ijЩ���۽��������γ��ȶ���ˮ���������
iii��ijЩ���ʵ��ܽ�ȣ�S��
T/�� | 20 | 40 | 60 | 80 | 100 |
S(Li2CO3)/g | 1.33 | 1.17 | 1.01 | 0.85 | 0.72 |
S(Li2SO4)/g | 34.7 | 33.6 | 32.7 | 31.7 | 30.9 |
I.�Ʊ�Li2CO3��Ʒ
��1������������Ϊ�ӿ컯ѧ��Ӧ���ʶ���ȡ�Ĵ�ʩ��____��
��2������2����Ҫ�ɷ���____��
��3������Һ2���ȼ���EDTA,�ټ��뱥��Na2C03��Һ,90���ַ�Ӧ��������� Li2CO3��Ʒ�IJ�����_______��
��4������lkg���3.50%�ķ�����﮵Ľ�����Ϊa��Li+ת��ΪLi2CO3��ת����Ϊb�����Ʒ�к�Li2CO3��������________g����Ħ������:Li2CO3 74 g.mol4)
II.����Li2CO3��Ʒ
��5����Li2CO3ת��ΪLiHCO3���ø�Ĥ�����LiHCO31��Һ�Ʊ��ߴ��ȵ�LiOH,��ת���õ�ؼ�Li2CO3�����ԭ����ͼ��ʾ�������ĵ缫��Ӧʽ��____,�ó�ʹ����_________(���������������������ӽ���Ĥ��
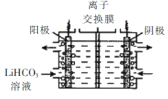
��.�Ʊ� LiFePO4
��6������ؼ�Li2CO3��C��FePO4�����·�Ӧ������LiFePO4��һ�ֿ�ȼ������,�÷�Ӧ�Ļ�ѧ����ʽ��_________��