��Ŀ����
��֪����A��B��C��GΪ���ֵ��ʣ�������A��B��G�����壬CΪ���壻��C��H��I��M����ͬһ��Ԫ�أ�
��D��K��L����̬���������D��������ˮ��L������ˮ����K������ˮ��
��F��һ����ɫҺ�壻
��M����ɫ��Ӧ�ʻ�ɫ��
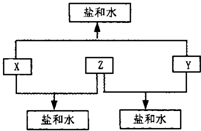
�⼸�����ʼ��ת����ϵ����ͼ��ʾ��
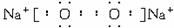
����д���¿հף�
��1��д��������D��J�Ļ�ѧʽ��D____________��J____________��
��2��������I�ĵ���ʽ��____________��L�ķ��ӵĿռ乹�ͣ�____________��
��3��д����Ӧ�ٵĻ�ѧ����ʽ��________________________��д����Ӧ�ڵ����ӷ���ʽ��________________________��
��4����Ӧ�����ڹ�ҵ����ʱ��Ŀǰ���õıȽ��Ƚ��ļ�����____________���Ӱ�ȫ���濼�����ɷ�ֹ________________________��
��1��HCl CaC2
��2��![]() ֱ����
ֱ����
��3��2Na2O2+2H2O====4NaOH+O2��
2Cl-+2H2O![]() H2��+Cl2��+2OH-
H2��+Cl2��+2OH-
��4�����ӽ���Ĥ�� ��������������H2��Cl2��ϱ�ը
�������ɢ�֪FΪH2O���ɢݢڢ�֪CΪNa��G�dz����µ����嵥�ʣ���ͼ��C��I��ת����֪GΪO2��HΪNa2O��IΪNa2O2��A��BΪ�����µ����嵥������ͼ��E+F��H2O��![]() A+B+M�������ȼҵ�ķ�Ӧԭ��֪EΪNaCl��MΪNaOH��A��B��H2��Cl2�е�һ�֣���DΪHCl��K��LΪ��̬�������KΪ��ȼ�����壬J��E��NaCl���ı�����Һ��Ӧ��������Һ��K��������Һͨ��L�ð�ɫ��������JΪCaC2��KΪC2H2��LΪCO2��CO2Ϊֱ���ͷ��ӡ�
A+B+M�������ȼҵ�ķ�Ӧԭ��֪EΪNaCl��MΪNaOH��A��B��H2��Cl2�е�һ�֣���DΪHCl��K��LΪ��̬�������KΪ��ȼ�����壬J��E��NaCl���ı�����Һ��Ӧ��������Һ��K��������Һͨ��L�ð�ɫ��������JΪCaC2��KΪC2H2��LΪCO2��CO2Ϊֱ���ͷ��ӡ�

 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д� ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д���֪
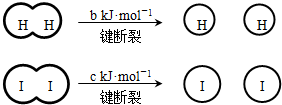 ��a��b��c�������㣩
��a��b��c�������㣩����˵������ȷ���ǣ�������
| A����Ӧ�������������������������� | B���Ͽ�1mol H-H����1mol I-I�������������ڶϿ�2mol H-I���������� | C���Ͽ�2mol H-I����������ԼΪ��c+b+a��kJ | D�����ܱ������м���2mol H2��2mol I2����ַ�Ӧ��ų�������С��2a kJ |

 ��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D2-������E2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��
��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D2-������E2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��
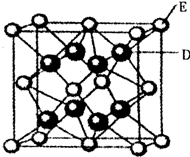 ��֪��A��B��C��D��E��F�����ڱ���ǰ36��Ԫ�أ�A��ԭ�Ӱ뾶��С��Ԫ�أ�BԪ�ػ�̬ԭ�ӵ�2P�����ֻ���������ӣ�CԪ�صĻ�̬ԭ��L��ֻ��2�ԳɶԵ��ӣ�D��Ԫ�����ڱ��е縺������Ԫ�أ�E2+�ĺ�������Ų���Arԭ����ͬ��F�ĺ˵������D��E�ĺ˵����֮�ͣ�
��֪��A��B��C��D��E��F�����ڱ���ǰ36��Ԫ�أ�A��ԭ�Ӱ뾶��С��Ԫ�أ�BԪ�ػ�̬ԭ�ӵ�2P�����ֻ���������ӣ�CԪ�صĻ�̬ԭ��L��ֻ��2�ԳɶԵ��ӣ�D��Ԫ�����ڱ��е縺������Ԫ�أ�E2+�ĺ�������Ų���Arԭ����ͬ��F�ĺ˵������D��E�ĺ˵����֮�ͣ�