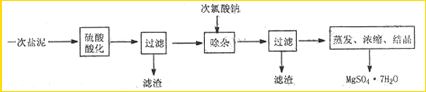
��Ŀ����
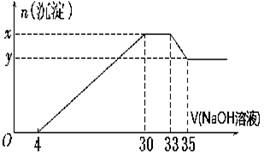
(10) ��200 mL��Mg2����Al3����NH��H����Cl�������ӵ���Һ�У���μ���5 mol��L��1������������Һ����������������Һ�����(mL)��������������ʵ���(mol)��ϵ����ͼ��ʾ��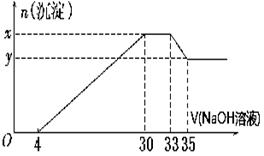
ͨ��������д���пհ�:
��1��. x��y�IJ�Ϊ
��2��. ԭ��Һ��c(Cl��)��
��3��. ԭ��Һ��pH��
��4��. ԭ��Һ��n(Mg2��)��n(Al3��)�� 
(5). ��������������Һ�������33mlʱ����Ӧ�����ӷ���ʽΪ .
(1).0.01 (2).0.825 (3). 1 (4). 5:1 (5).Al(OH)3 + OH-- ="=" AlO2-- + 2H2O
����

��ϰ��ϵ�д�
�����Ŀ