��Ŀ����
Ag+Ũ��Ϊ0.100 mol��L-1����Һ5 mL����������ʵ�����ij������Σ���ַ�Ӧ������±��������������ˡ�ϴ�Ӻ���200 W�����º�ɣ��õ�1.297��10��1�����±�������ΪAgX�������ʵ���Ϊ________ mol���������ݷ������������Ƿ�ΪAgX����________����ǡ����ǡ���,��ΪAgX�����������Ļ�ѧʽΪ________��������ΪAgX�������˿ղ����
(2)���±�������Ϊ�����±�����Ħ������Ϊ________ g��mol-1�����ݷ��������Ļ�ѧʽΪ________��
(1)0.000 5 ����
(2)26 LiF
����:��1����ϵʽAg+��AgX,n(AgX)=n(Ag+)=0.100mol��L-1��5mL��10![]() =
=

��ϰ��ϵ�д�
�����Ŀ
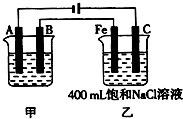 ͼΪ������ļס��������أ��Իش�
ͼΪ������ļס��������أ��Իش�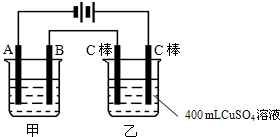 ��ͼΪΪ�����ļס��������أ��Իش�
��ͼΪΪ�����ļס��������أ��Իش�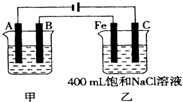 ��ͼΪ������ļס��������أ��Իش�
��ͼΪ������ļס��������أ��Իش�